Abstract
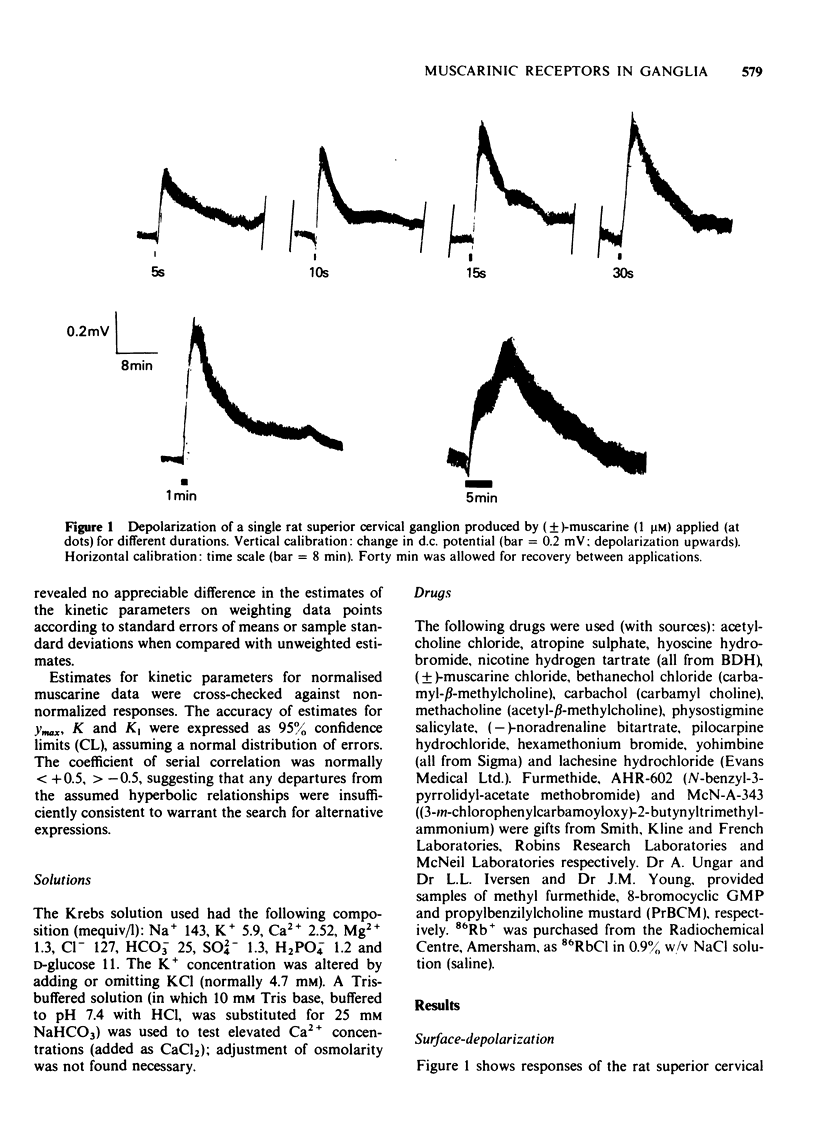
1 Potential changes in isolated superior cervical ganglia of the rat produced by muscarinic-receptor agonists were recorded by an extracellular `air-gap' method.
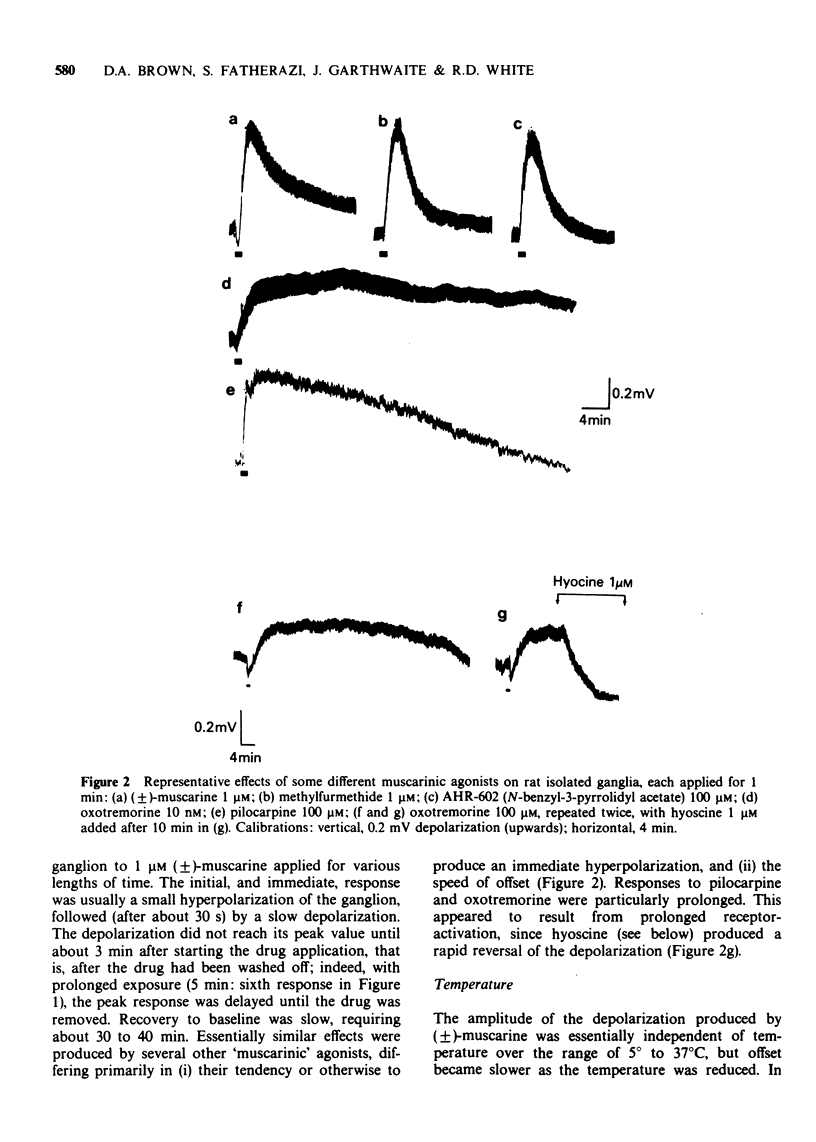
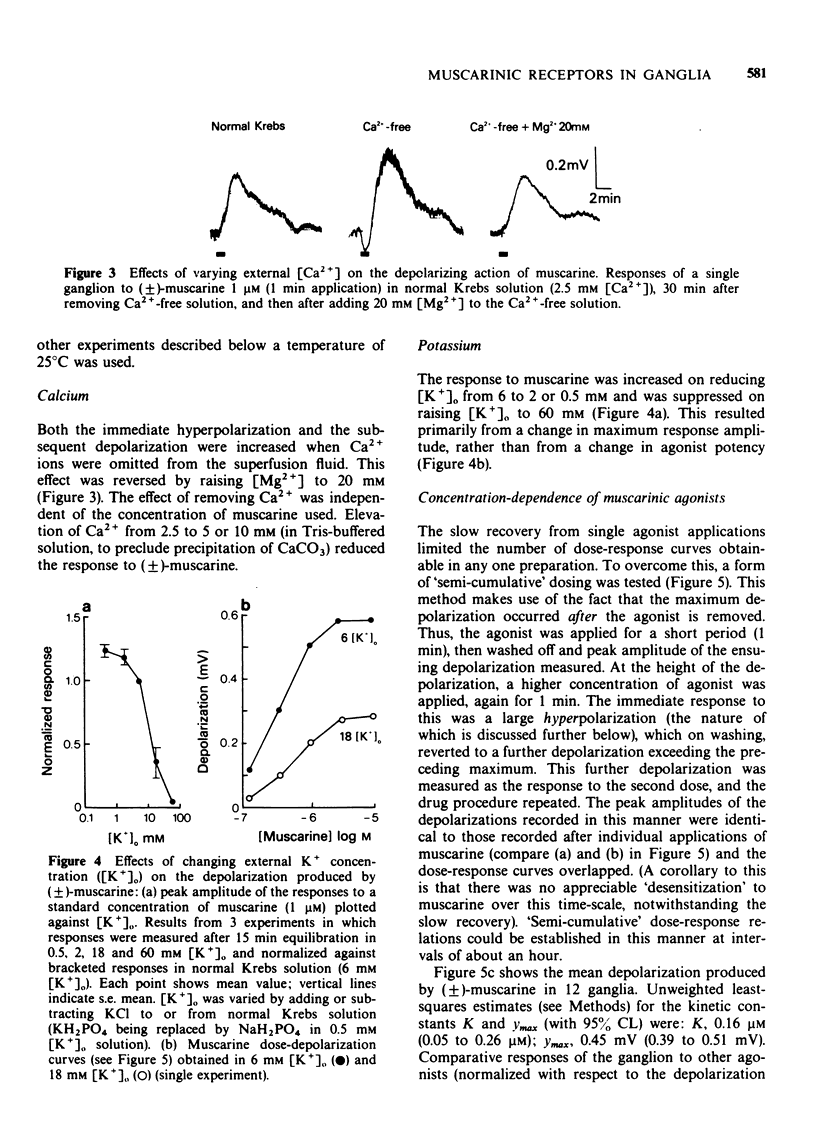
2 Muscarinic agonists produced a delayed low-amplitude ganglion depolarization, frequently preceded by a hyperpolarization. Potentials were enhanced by reducing [K+]o or [Ca2+]o.
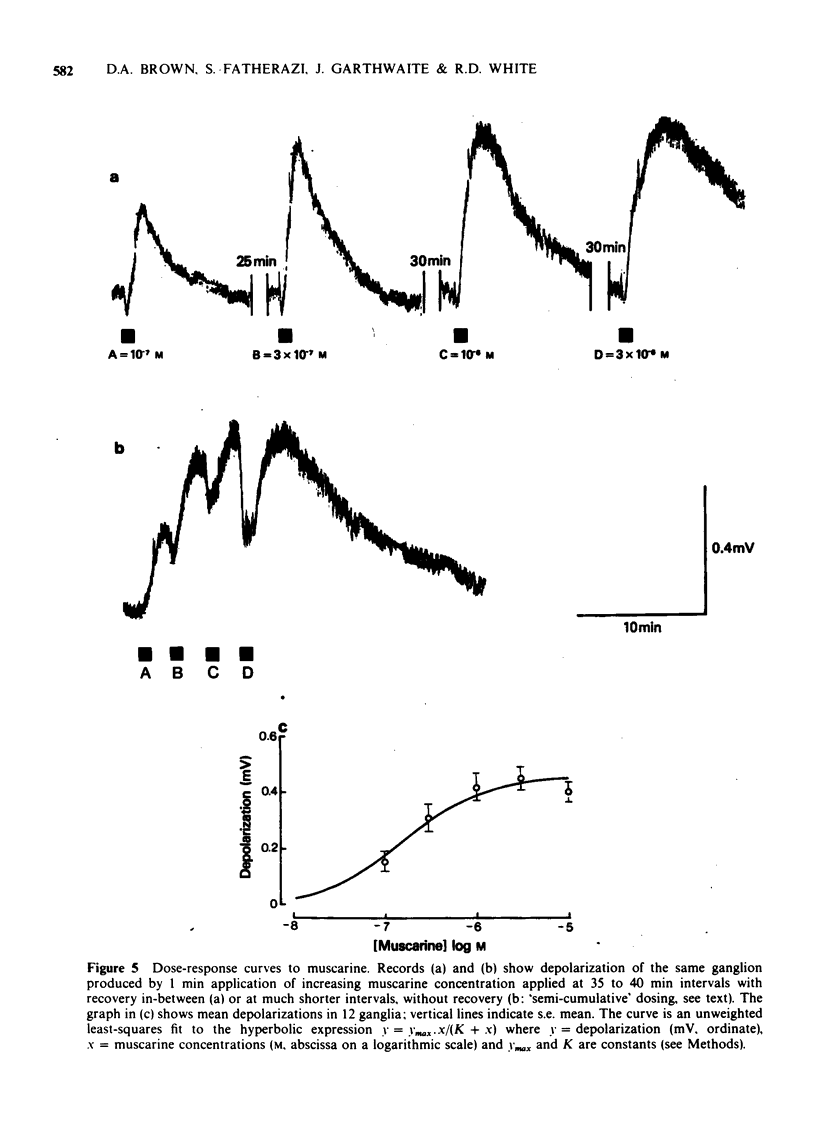
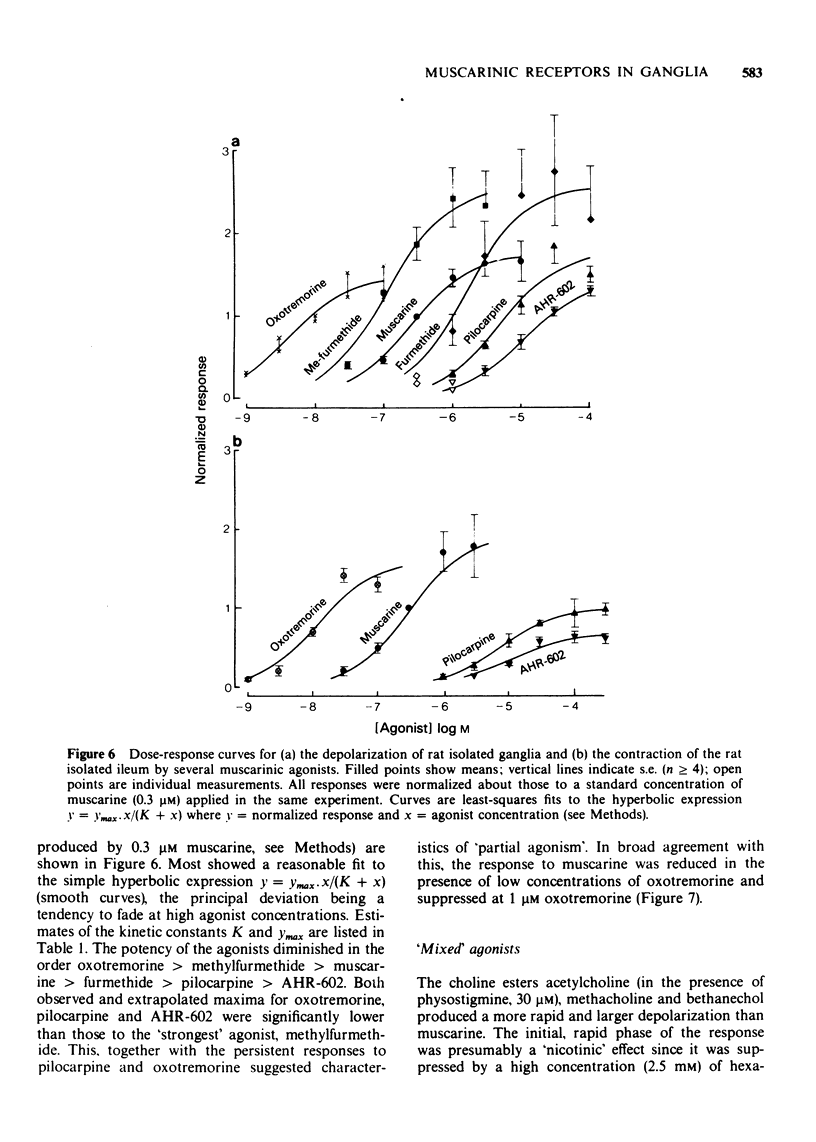
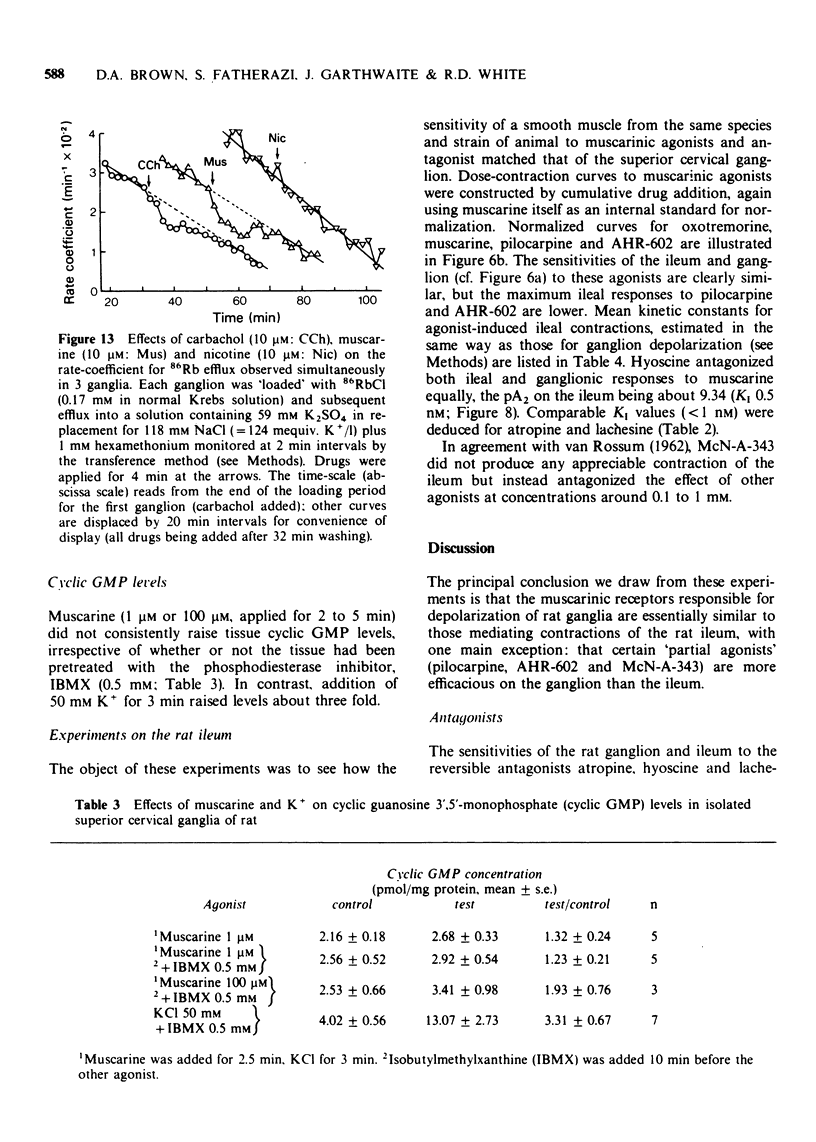
3 Mean ED50 values (μM) for depolarization at 25°C were: oxotremorine 0.004, methylfurmethide 0.11, (±)-muscarine 0.24, furmethide 1.56, pilocarpine 4.81 and AHR-602 (N-benzylpyrrolidylacetate methobromide) 10.8. Responses produced by oxotremorine, pilocarpine and AHR-602 showed some characteristics of `partial agonism'. ED50 values (μM) for choline esters (measured in the presence of 2.5 mM hexamethonium) were: acetylcholine 3.2, methacholine 59 and bethanechol 78.
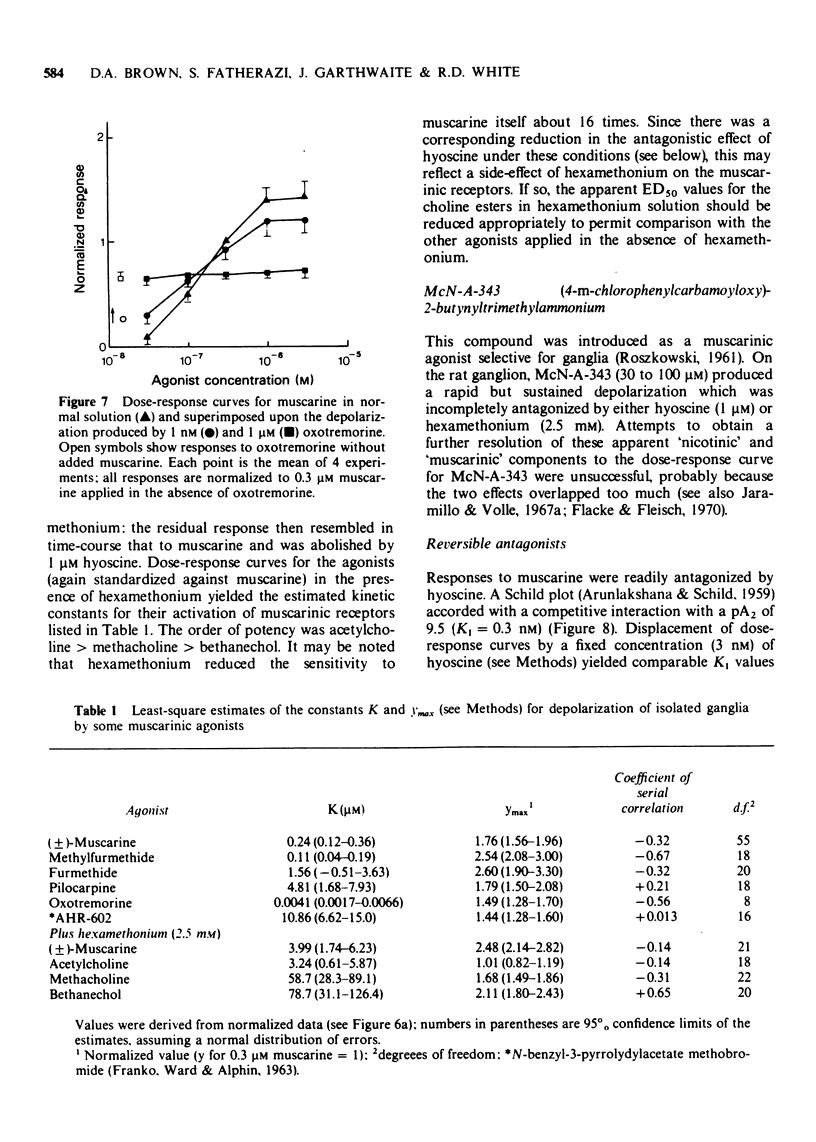
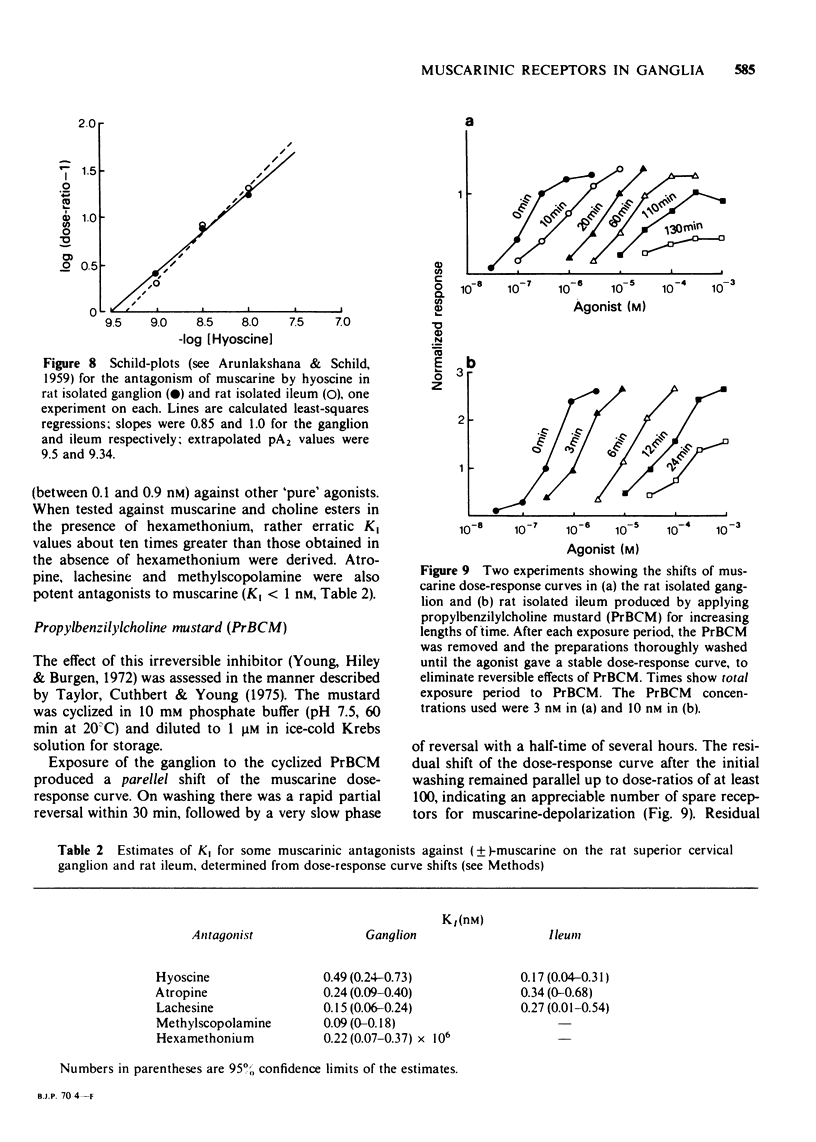
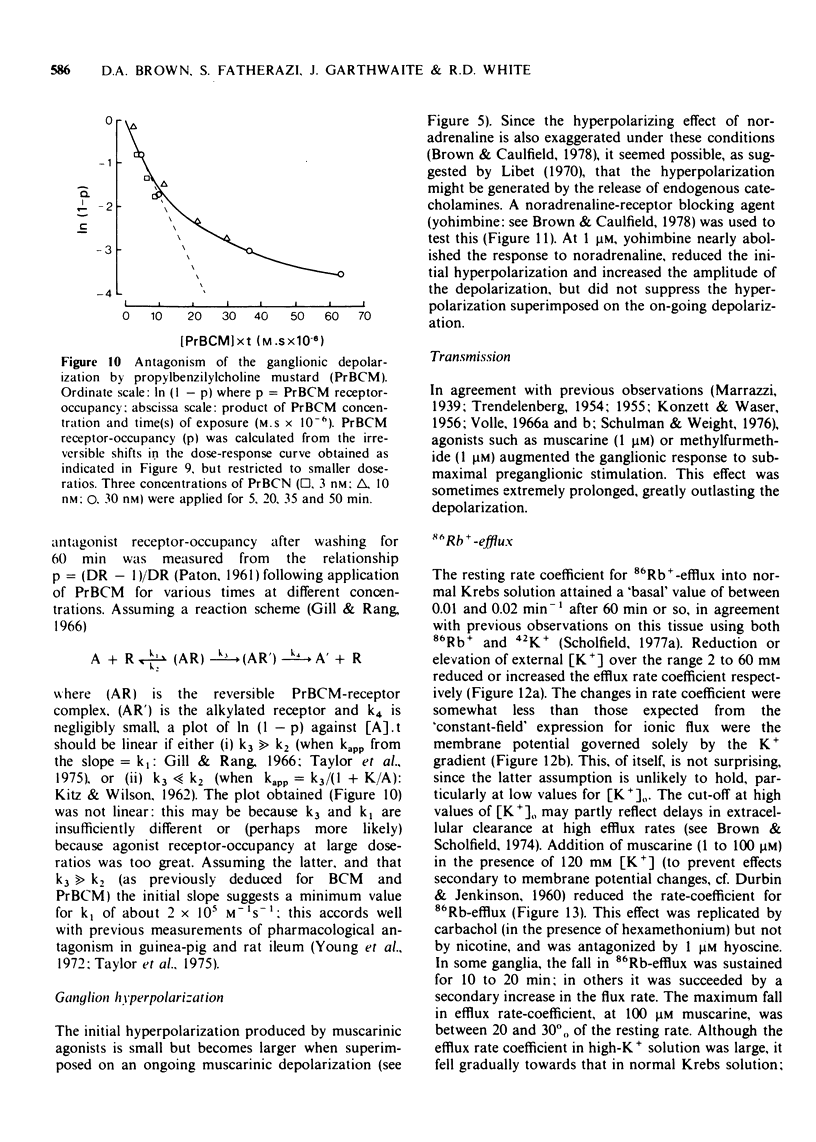
4 Responses to muscarine were antagonized by hyoscine (KI 0.49 nM) atropine (KI 0.24 nM) methylscopolamine (KI 0.09 nM) lachesine (KI 0.15 nM) and (weakly) by hexamethonium (KI 0.2 mM). Propylbenzilylcholine mustard produced irreversible antagonism with an apparent onset rate constant of 2 × 105 M-1S-1.
5 Depolarization was accompanied by facilitation of submaximal ganglionic transmission.
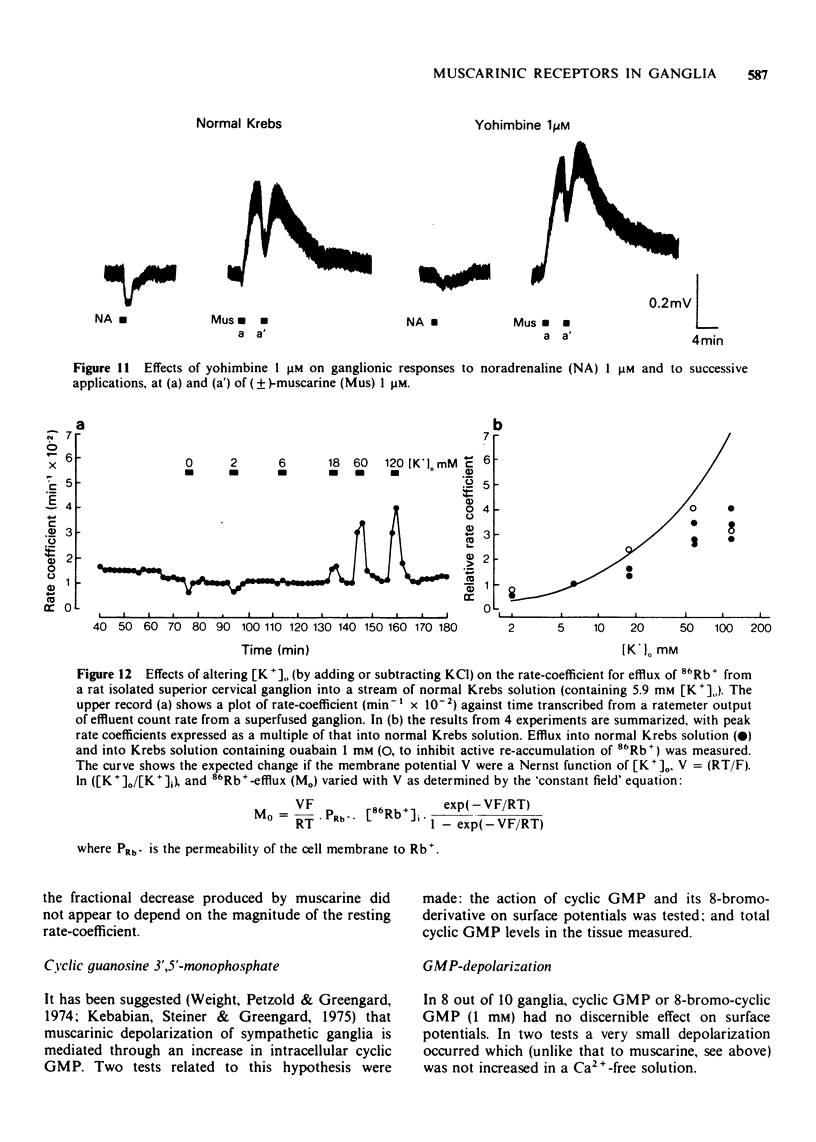
6 Muscarine (1 to 100 μM) initially reduced, then increased, the rate of 86Rb+-efflux from isolated ganglia at both 6 and 120 mM [K+]o. These effects were reduced by 1 μM hyoscine.
7 No consistent change in the amounts of cyclic 3′,5′-guanosine monophosphate in isolated ganglia accompanying muscarinic depolarization could be detected.
8 Mean against ED50 values (μM) for contracting the rat isolated ileum were: oxotremorine 0.012, methylfurmethide 0.29, (±)-muscarine 0.48, pilocarpine 7.8 and AHR-602 9.9. Mean antagonist KI values (nM) were: hyoscine 0.17, atropine 0.34 and lachesine 0.27.
9 It is concluded that ganglionic muscarinic receptors are quite similar to ileal receptors in terms of agonist ED50 and antagonist KI values, and that the major difference between them lies in the greater `efficacy' of certain agonists (pilocarpine, AHR-602 and McN-A-343) on the ganglion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N., PERRY W. L., ROBERTSON P. A. The effect of muscarine on perfused superior cervical ganglia of cats. Br J Pharmacol Chemother. 1956 Dec;11(4):442–448. doi: 10.1111/j.1476-5381.1956.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N. The nicotinic action of substances supposed to be purely smooth-muscle stimulating; effect of BaCl2 and pilocarpine on the superior cervical ganglion. J Physiol. 1949 Dec 15;110(1-2):164–172. doi: 10.1113/jphysiol.1949.sp004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBACHE N. The nicotinic action of substances supposed to be purely smooth-muscle stimulating; effects of alpha-beta-ethylal-gamma-trimethylammoniumpropanediol (2268 F) upon skeletal muscle and ganglion cells. J Physiol. 1949 Dec 15;110(1-2):145–163. doi: 10.1113/jphysiol.1949.sp004428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Berry K. J., Glenton P. A., Nilolaou N. M., Soh K. S. A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29 degrees C and in ileum at 29 degrees C and 37 degrees C. Br J Pharmacol. 1976 Dec;58(4):613–620. doi: 10.1111/j.1476-5381.1976.tb08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C. The binding of agonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):723–736. [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. Effects of hexamethonium and hyoscine on the drug-induced depolarization of isolated superior cervical ganglia. Br J Pharmacol Chemother. 1966 Mar;26(3):521–537. doi: 10.1111/j.1476-5381.1966.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Garthwaite J. Intracellular pH and the distribution of weak acids and bases in isolated rat superior cervical ganglia. J Physiol. 1979 Dec;297(0):597–620. doi: 10.1113/jphysiol.1979.sp013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. A very simple method for recording ganglion depolarization. J Physiol. 1975 Mar;246(2):24P–26P. [PubMed] [Google Scholar]
- Brown D. A., Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978 Nov 3;156(1):187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Movements of labelled sodium ions in isolated rat superior cervical ganglia. J Physiol. 1974 Oct;242(2):321–351. doi: 10.1113/jphysiol.1974.sp010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Nicotine washout rates from isolated rat ganglia in relation to recovery from nicotine depolarization. Br J Pharmacol. 1972 May;45(1):29–36. doi: 10.1111/j.1476-5381.1972.tb09573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R. Muscarinic receptor binding in rat sympathetic ganglia is unaffected by denervation. Brain Res. 1978 Mar 31;143(3):573–579. doi: 10.1016/0006-8993(78)90370-0. [DOI] [PubMed] [Google Scholar]
- Busis N. A., Weight F. F., Smith P. A. Synaptic potentials in sympathetic ganglia: are they mediated by cyclic nucleotides? Science. 1978 Jun 2;200(4345):1079–1081. doi: 10.1126/science.206964. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The acetylcholine receptors of Renshaw cells. Exp Brain Res. 1966;2(1):66–80. doi: 10.1007/BF00234361. [DOI] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. H., Laidlaw P. P. The significance of the suprarenal capsules in the action of certain alkaloids. J Physiol. 1912 Aug 2;45(1-2):1–26. doi: 10.1113/jphysiol.1912.sp001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Dodd J., Kelly J. S. Ach-evoked excitation of cortical neurones [proceedings]. J Physiol. 1977 Dec;273(2):79P–80P. [PubMed] [Google Scholar]
- Dun N. J., Kaibara K., Karczmar A. G. Muscarinic and cGMP induced membrane potential changes: differences in electrogenic mechanisms. Brain Res. 1978 Jul 21;150(3):658–661. doi: 10.1016/0006-8993(78)90833-8. [DOI] [PubMed] [Google Scholar]
- Flacke W., Fleisch J. H. The effect of ganglionic agonists and antagonists on the cardiac sympathetic ganglia of the dog. J Pharmacol Exp Ther. 1970 Jul;174(1):45–55. [PubMed] [Google Scholar]
- Gill E. W., Rang H. P. An alkylating derivative of benzilylcholine with specific and long-lasting parasympatholytic activity. Mol Pharmacol. 1966 Jul;2(4):284–297. [PubMed] [Google Scholar]
- Hashiguchi T., Ushiyama N. S., Kobayashi H., Libet B. Does cyclic GMP mediate the slow excitatory synaptic potential in sympathetic ganglia? Nature. 1978 Jan 19;271(5642):267–268. doi: 10.1038/271267a0. [DOI] [PubMed] [Google Scholar]
- Henderson C. G., Ungar A. Antagonist affinity constants for adrenomedullary muscarinic receptors [proceedings]. Br J Pharmacol. 1977 Mar;59(3):499P–500P. [PMC free article] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Burgen A. S., Mehta P. The binding of antagonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):737–750. [PubMed] [Google Scholar]
- Jaramillo J., Volle R. L. Ganglion blockade by muscarine, oxotremorine and AHR-602. J Pharmacol Exp Ther. 1967 Oct;158(1):80–88. [PubMed] [Google Scholar]
- Jaramillo J., Volle R. L. Nonmuscarinic stimulation and block of a sympathetic ganglion by 4-(m-chlorophenylcarbamoyloxy)-2-butynyltrimethylammonium chloride (McN-A-343). J Pharmacol Exp Ther. 1967 Aug;157(2):337–345. [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- KONZETT H., WASER P. G. Zur ganglionären Wirkung von Muscarin. Helv Physiol Pharmacol Acta. 1956;14(2):202–206. [PubMed] [Google Scholar]
- Kebabian J. W., Steiner A. L., Greengard P. Muscarinic cholinergic regulation of cyclic guanosine 3,5-monophosphate in autonomic ganglia: possible role in synaptic transmission. J Pharmacol Exp Ther. 1975 May;193(2):474–488. [PubMed] [Google Scholar]
- Krnjević K. Central cholinergic pathways. Fed Proc. 1969 Jan-Feb;28(1):113–120. [PubMed] [Google Scholar]
- Krnjević K., Phillis J. W. Pharmacological properties of acetylcholine-sensitive cells in the cerebral cortex. J Physiol. 1963 May;166(2):328–350. doi: 10.1113/jphysiol.1963.sp007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Ionic mechanism of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Brain Res. 1974 Dec 6;81(2):338–342. doi: 10.1016/0006-8993(74)90949-4. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. The muscarinic effects of acetylcholine on the action potential of bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):703–716. doi: 10.2170/jjphysiol.26.703. [DOI] [PubMed] [Google Scholar]
- LEVY B., AHLQUIST R. P. A study of sympathetic ganglionic stimulants. J Pharmacol Exp Ther. 1962 Aug;137:219–228. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- McAfee D. A., Greengard P. Adenosine 3',5'-monophosphate: electrophysiological evidence for a role in synaptic transmission. Science. 1972 Oct;178(58):310–312. doi: 10.1126/science.178.4058.310. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- ROSZKOWSKI A. P. An unusual type of sympathetic ganglionic stimulant. J Pharmacol Exp Ther. 1961 May;132:156–170. [PubMed] [Google Scholar]
- Scholfield C. N. Movements of radioactive potassium in isolated rat ganglia. J Physiol. 1977 Jun;268(1):123–137. doi: 10.1113/jphysiol.1977.sp011850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. A., Weight F. F. Synaptic transmission: long-lasting potentiation by a postsynaptic mechanism. Science. 1976 Dec 24;194(4272):1437–1439. doi: 10.1126/science.188131. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. The action of histamine and pilocarpine on the superior cervical ganglion and the adrenal glands of the cat. Br J Pharmacol Chemother. 1954 Dec;9(4):481–487. doi: 10.1111/j.1476-5381.1954.tb00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENDELENBURG U. The potentiation of ganglionic transmission by histamine and pilocarpine. J Physiol. 1955 Aug 29;129(2):337–351. doi: 10.1113/jphysiol.1955.sp005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. K., Cuthbert A. W., Young M. Muscarinic receptors in rat intestinal muscle: comparison with the guinea pig. Eur J Pharmacol. 1975 Apr;31(2):319–326. doi: 10.1016/0014-2999(75)90055-2. [DOI] [PubMed] [Google Scholar]
- Ward D., Young J. M. Ligand binding to muscarinic receptors in intact longitudinal muscle strips from guinea-pig intestine. Br J Pharmacol. 1977 Oct;61(2):189–197. doi: 10.1111/j.1476-5381.1977.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Petzold G., Greengard P. Guanosine 3',5'-monophosphate in sympathetic ganglia: increase assoicated with synaptic transmission. Science. 1974 Dec 6;186(4167):942–944. doi: 10.1126/science.186.4167.942. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M., Hiley R., Burgen A. S. Homologues of benzilylcholine mustard. J Pharm Pharmacol. 1972 Dec;24(12):950–954. doi: 10.1111/j.2042-7158.1972.tb08925.x. [DOI] [PubMed] [Google Scholar]


