Abstract
In areas of stable malaria transmission, susceptibility to Plasmodium falciparum malaria increases during first pregnancy. Women become resistant to pregnancy malaria over successive pregnancies as they acquire antibodies against the parasite forms that sequester in the placenta, suggesting that a vaccine is feasible. Placental parasites are antigenically distinct and bind receptors, like chondroitin sulfate A (CSA), that are not commonly bound by other parasites. We used whole-genome-expression analysis to find transcripts that distinguish parasites of pregnant women from other parasites and employed a novel approach to define and adjust for cell cycle timing of parasites. Transcription of six genes was substantially higher in both placental parasites and peripheral parasites from pregnant women, and each gene encodes a protein with a putative export sequence and/or transmembrane domain. This cohort of genes includes var2csa, a member of the variant PfEMP1 gene family previously implicated in pregnancy malaria, as well as five conserved genes of unknown functions. Women in East Africa acquire antibodies over successive pregnancies against a protein encoded by one of these genes, PFD1140w, and this protein shows seroreactivity similar to that of VAR2CSA domains. These findings suggest that a suite of genes may be important for the genesis of the placental binding phenotype of P. falciparum and may provide novel targets for therapeutic intervention.
By adulthood, individuals living in areas of stable malaria transmission acquire clinical immunity as a result of repeated infection with Plasmodium falciparum (11, 41, 42). However, women pregnant for the first time become susceptible to infection by antigenically distinct parasites that sequester in the placenta (6, 17), producing a specific malaria syndrome known as placental malaria, pregnancy malaria, or pregnancy-associated malaria. Pregnancy malaria commonly causes severe maternal anemia, low birth weight, and increased neonatal and infant mortality. The deaths of between 100,000 and 200,000 African infants each year are attributed to malaria during pregnancy, making this an enormous public health burden (27).
Placental parasites have distinct properties that suggest a unique repertoire of surface antigens. Infected erythrocytes (IE) in the placenta adhere to low-sulfated forms of chondroitin sulfate A (CSA), a glycosaminoglycan found on the surface of the syncytiotrophoblast (1, 2, 20, 22, 25) and throughout the intervillous spaces of infected placentas (47). Although the distribution of CSA on vascular surfaces has not been fully defined, CSA binding appears to be largely associated with parasites from pregnant women (18, 57). Conversely, placental parasites do not typically bind CD36, ICAM-1, or other endothelial receptors (17, 57) that commonly support binding of parasites associated with other malaria syndromes (5, 6, 28, 64).
The frequency and severity of placental malaria decrease over successive pregnancies, as women acquire adhesion-blocking antibodies against placental IE (21). Serum immunoglobulin G (IgG) from multigravid women living in areas of endemicity has been shown to block adhesion of placental or CSA-selected parasites collected from different continents (18, 21, 54, 66). Adhesion-blocking antibodies are not detected in males or women before first pregnancy. This pattern of naturally acquired immunity is consistent with repeated exposure to a finite number of antigens during pregnancy that are not seen in childhood infections, raising expectations that a vaccine can be developed once the IE surface antigens of placental parasites are identified.
The search for IE surface antigens of placental parasites has focused on the var gene family. var genes encode antigenically varied 200- to 400-kDa multidomain IE surface proteins called PfEMP1 that have been implicated in adhesion to other receptors (62, 65). There are 59 var genes in the 3D7 P. falciparum genome (24) and probably similar numbers in other strains (33).
var gene variants varCS2 (53) and FCR3.varCSA (var1csa) (10) have both been linked to CSA binding in studies of parasites selected in vitro. Deletion of FCR3.varCSA resulted in initial loss of CSA binding, suggesting a role for VAR1CSA in binding of the FCR3 parasite line to CSA. However, other studies suggest that var1csa is not central to adhesion of placental parasites. Transcription of var1csa is not restricted to placental parasites (19), and seroreactivity to expressed VAR1CSA is not parity related (31, 50). After selection on CSA, the FCR3 knockout parasite regained binding to CSA and expressed a novel PfEMP1 gene, subsequently shown to be var2csa (3, 23).
A comprehensive analysis of var gene transcription in CSA-selected NF54 laboratory parasites revealed that PFL0030c, now known as var2csa, was transcribed at high levels (60). Transcription of var2csa has been confirmed in other CSA-selected laboratory strains as well as isolates from pregnant women (13, 15). var2csa was recently shown to be the most highly expressed var gene detected in placental parasites from Malawi, although one placental parasite sample preferentially expressed the DBL2γ domain of varCS2 (14). CSA-selected parasites expressing var2csa are recognized by sera from Ghana and Senegal in a parity- and gender-specific manner (60, 67) that correlates with protection.
The mass of data accumulated over the last few years is consistent with the hypothesis that VAR2CSA is the dominant PfEMP1 associated with parasite adhesion to the placenta. However, fundamental gaps remain in our understanding of placental parasites. While VAR2CSA has been shown to be localized to the IE cell surfaces of CSA-selected parasites (4, 15), there are no published reports of VAR2CSA cell surface localization in parasites from pregnant women. Cross-linking experiments examining binding of IE surface proteins to CSA showed that an unidentified 22-kDa protein and not VAR2CSA bound to CSA (26), and no studies have demonstrated that soluble VAR2CSA can inhibit binding of placental parasites. These findings raise the possibility that other proteins in addition to VAR2CSA are required for the placental parasite phenotype, for example, as part of a multimeric protein complex at the IE surface (26), which could also reconcile the biochemical properties of PfEMP1 with its membrane topology (51). PfEMP1 is synthesized as a peripheral membrane protein (51) and appears to be part of a complex, rather than in vesicles in the IE cytosol (32), prior to localization to the Maurer's cleft and subsequent insertion in the erythrocyte membrane (35, 43, 51).
Since the erythrocyte lacks a protein transport apparatus, many of the necessary components must be exported by the parasite. The predicted secretome responsible for erythrocyte remodeling, protein transport, and adhesion properties is composed of about 400 proteins with sorting motifs that direct proteins beyond the parasitophorous vacuole membrane plus additional proteins that lack these motifs (7, 12, 29, 30, 44, 48, 63). Many secretome proteins are encoded by members of paralogous families with unknown functions that show dramatic expansion in P. falciparum and may define specific virulence properties.
In an effort to characterize the placenta binding phenotype of clinical parasites and to identify novel antigens that are expressed by parasites causing infections, we examined the whole-genome transcriptional profiles of parasites collected from Tanzanian women with pregnancy malaria. Parasites were profiled either directly after collection in the clinic or after a maximum of 20 h in culture so that gene expression patterns characteristic of parasites causing disease would be maintained. Both circulating early-stage parasites and sequestered late-stage parasites from women with pregnancy malaria were analyzed so that the transcriptional profiles of parasites just prior to and during sequestration could be determined. We found that increased var2csa expression is always observed in maternal parasites at both stages of intraerythrocytic development and that a suite of five additional genes, all with unknown functions, is also consistently up-regulated by these parasites.
MATERIALS AND METHODS
Clinical sample collection and parasite adhesion assays.
All samples were collected from study participants in the ongoing Mother Offspring Malaria Studies (MOMS) Project carried out in Muheza district, northeastern Tanzania, an area of intense malaria transmission as previously described (16, 46). Women provided signed, informed consent for themselves and their children to participate in the study and to provide samples for parasite studies. Study procedures involving human participants were approved by the International Clinical Studies Review Committee of the Division of Microbiology and Infectious Diseases at the U.S. National Institutes of Health, and ethical clearance was obtained from the Institutional Review Board of Seattle Biomedical Research Institute and the National Medical Research Coordinating Committee in Tanzania.
Placental parasites were obtained as previously described (17). Parasites from the peripheral circulation of mothers and children were cultured and tested for adhesion to immobilized purified receptors, including CSA, hyaluronic acid (HA), thrombospondin (TSP), ICAM-1, CD36, and bovine serum albumin (BSA) (control). Assays were performed within 24 h of sample collection (16).
RNA preparation and microarray hybridization.
Intervillous blood was obtained by mechanical grinding of placentas, and intraerythrocytic parasites were released from red blood cells by saponin lysis. The released parasites were stored in RNA Later solution (Ambion) at −20°C and subsequently solubilized in TRIzol (Invitrogen). Peripheral parasites from mothers and children were collected and stored in either TRIzol or RNA Wiz (Ambion) and stored at −80°C. Peripheral parasites from children were cultured for 20 h until parasites matured to the trophozoite stage, solubilized in TRIzol, and stored at −80°C.
RNA was isolated according to the manufacturer's instructions. Microarray probes were prepared using a MessageAmpII aminoallyl labeling kit, starting with 1 to 3 μg of total RNA from each sample, following the kit instructions (Ambion). Probes were coupled to Cy3 and Cy5 monoesters (GE Healthcare Biosciences). Probe samples to be compared were combined and fragmented with RNA fragmentation reagent (Ambion).
All slides were prehybridized at 63°C with 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% sodium dodecyl sulfate (SDS), 0.1 mg/ml BSA for 60 min to 4 h and then washed in nuclease-free water and dried. The hybridization buffer was 3× SSC, 25 mM HEPES (pH 7.5), 0.75 mg/ml poly(A) DNA (Sigma), 0.2% SDS. Differential gene expression was determined by direct comparison of 5 μg of a labeled probe from maternal-parasite RNA to 5 μg of a labeled probe with comparable specific activity from child-derived-parasite RNA. Multiple comparisons were made for each clinical parasite sample. Slides were hybridized at 63°C for 16 to 20 h and washed in 2× SSC, 0.2% SDS, followed by a wash in 0.1× SSC.
Data were acquired with GenePix Pro 4000A (Molecular Devices) and analyzed using GenePix 5.1 (Axon Instruments) and Acuity 4.0 (Molecular Devices) software. Data were normalized using a locally weighted linear regression algorithm without background subtraction. Unflagged spots whose background-subtracted intensities were 3 standard deviations above the local background level were analyzed.
Microarray fabrication.
Microarrays were prepared by spotting the P. falciparum genome set of 70-mer oligonucleotides (Operon Biotechnologies) and 396 PfEMP1 70-mer oligonucleotides. These include the primary 3D7 elements from the Operon set, additional domain-specific elements designed for selected 3D7 var genes, elements from sequences obtained from field studies, the conserved regions from type A PfEMP1 genes implicated in severe malaria (37), and consensus var2csa elements designed by aligning 3D7, IT4, and DD2 strains of P. falciparum. In total, 4,872 3D7 P. falciparum genes were represented by oligonucleotide probes. Custom oligonucleotides were designed manually or using OligoArraySelector software and then synthesized (Illumina). All oligonucleotides were resuspended in 3× SSC to a concentration of 50 μΜ and spotted in duplicate on each UltraGapII slide (Corning), using a Gene Machine GR-04 Omnigrid oligonucleotide arrayer (Gene Machines). Slides were cross-linked and blocked using protocols from http://derisilab.ucsf.edu.
Quantitative reverse transcription-PCR (Q-PCR).
DNA was removed from RNA with Turbo DNase (Ambion), following the kit instructions. RNA was reverse transcribed for 2 h at 46°C using Superscript III (Invitrogen) and primed with random nonamers (Sigma Aldrich). RNA from ring stage parasites was prepared by amplification using a Message Amp II kit (Ambion). PCR primers for selected genes were designed using Primer3 software (58). Real-time PCR was performed with the 7500 fast real-time PCR system (Applied Biosystems) for 45 cycles at 59οC using a SYBR green master mix kit (Applied Biosystems) and 0.5 mM primers. PCRs were performed in triplicate. Relative quantitation of RNA expression was done using the ΔΔCT method (user bulletin 2; Applied Biosystems), using seryl-tRNA synthetase and fructose biphosphate aldolase as constitutive controls (60). Maternal samples were compared to samples from children, which served as the calibrator. P values were determined using the Kruskal-Wallis algorithm. Primer pairs used in this study are listed in Table 1.
TABLE 1.
Real-time-PCR primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| PFB0115w | 5′-AAGGTATACCAAAGGAACAACC-3′ | 5′-CATCAACATGTGGTTCTTGTC-3′ |
| PFB0115w | 5′-AGAACAACAGAATTATCAACTACATC-3′ | 5′-TGTTCCTTTGGTATACCTTGTG-3′ |
| PFD1140w | 5′ATGAAGCGTTGAATCTTTTATC-3′ | 5′-TTTTCACTCTTACATAATTCATTCC-3′ |
| PFD1140w | 5′-GATATTGATGATATTGAAGAAGAGG-3′ | 5′-AAATCATTATAAGTTCCGGATG-3′ |
| PFI1785w | 5′-ATCTGCAGGTAGATATTCATGG-3′ | 5′-AAATTCTTAAAGCGGTTTTACC-3′ |
| PFI1785w | 5′-GAAATGAAGAAAGGAAATTTAGC-3′ | 5′-TTTTAAGGGTTTCTTCACATTC-3′ |
| PFF0435 | 5′-CAAGGAGAAGCTGGTGTTATAG-3′ | 5′-AACCTGTTTGTACTTCATCTGC-3′ |
| PFF0435 | 5′-TGATAAATTAGGTGCTCCATTC-3′ | 5′-AGATATCCCATACGTTGACAAG-3′ |
| PFL0050c | 5′-AAATTCAGTAAGAATTGCATGG-3′ | 5′-TCATCTACGTCTTCAATTTCATC-3′ |
| PFL0050c | 5′-ATGAATGTCGTCATATTGTTCC | 5′-AGCTTTCCATGCAATTCTTAC-3′ |
| MAL7P1.225 | 5′-TAGCTAAAGATGGATTTGATGG-3′ | 5′-TTGATTTATACCATGTTTCACG-3′ |
| MAL7P1.225 | 5′-ATGGATTTGATGGTATGTTGAC-3′ | 5′-TGATGGTATGTTGACAGAATTATC-3′ |
| PF10-0013 | 5′-TTTAGGAGATTCATTGAAGAGAAC-3′ | 5′-TCAGCATATCTTTCGTTCATAAG-3′ |
| PF10-0013 | 5′-ATATCGGAACAAACTTCTGATG-3′ | 5′-GATTGTAATTCATGATTGTCCTC-3′ |
| PF07_0073 | 5′- AAGTAGCAGGTCATCGTGGTT-3′ | 5′-TTCGGCACATTCTTCCATAA-3′ |
| PF14_0425 | 5′-TGTACCACCAGCCTTACCAG-3′ | 5′-TTCCTTGCCATGTGTTCAAT-3′ |
Cell cycle mapping.
Hourly microarray studies encompassing the 48-h HB3 blood stage cycle (8) were used to map the developmental timing of clinical samples. We determined that 1,847 elements had valid spots across 51 of 55 microarrays in the HB3 series (i.e., all arrays other than TP_1a, TP_7b, TP_11a, and TP_39). The intensity of each element at each time point was divided by total intensity of Cy5 for the corresponding time point, and the logarithm was taken from the result of the division. Clinical sample microarrays were treated similarly. Elements common to both datasets were used to calculate both Euclidean and correlation distances between datasets. Correlation distances were defined as 1 minus the correlation coefficient. Euclidean distances were centered by deducting the mean of all 51 distances and plotted.
Multiple regression analysis.
For each probe, the log2 intensities of all samples were calculated from microarray ratios by using multiple regression analysis. The design matrix was designated with values 1 and −1 in positions where samples were arrays in Cy5 and Cy3, respectively. P values corrected for multiple testing were calculated according to the Westfall-Young algorithm (69).
Hierarchical clustering of timing profiles.
Timing profiles in correlation distances from different microarray slides from the same clinical samples were calculated, and their means were used for cluster analysis. Hierarchical clustering was performed using Euclidean distances and an average linkage algorithm.
PCA of the blood stage cycle.
The 51 HB3 blood stage microarrays with valid spots (see above), plus all clinical microarrays from the current study, were normalized by total intensity and log transformed. If valid signals for a particular element existed in at least half of the arrays for a clinical sample, their mean value was used as the intensity of the element. Between 51 blood stage arrays and 20 clinical samples, there was a data set of 1,554 shared elements. Principal-component analysis (PCA) of the 51 blood stage time points (restricted to the described 1,554-element data set) showed that the first three components can be retained, and they explain 93% of the variance. After varimax rotation, these three principal components corresponded to the late ring-early trophozoite, late trophozoite-early schizont, and late schizont-early ring stages (see Fig. S3 in the supplemental material). Loading of clinical samples on the principal components was calculated as a correlation coefficient between each clinical sample and the principal components.
PfEMP1 gene expression analysis.
All microarray elements were blasted against 97 PfEMP1 genes, pseudogenes, and truncated genes from the 3D7 strain. Partial homology with an E score of 6e−10 or less was considered a hit (see Table S3 in the supplemental material). Three hundred ninety-six elements were selected. The intensity of each element in each array was divided by the total intensity for the corresponding array. If the intensities of duplicate spots differed twofold or more, both spots were considered invalid. Otherwise, their average value was taken. If any array had an element whose intensity differed more than threefold from the mean intensity of the element for that clinical sample across all applicable arrays, then that element was excluded from further analysis. After this step, 377 elements had consistent measurements across all clinical samples. The switched-off expression level of a particular element was calculated as the mean expression level of the three lowest-expressing clinical samples (out of 11) of the same element. As a compromise between sensitivity in detection of switched-on expression and reduction of false scoring due to low-homology cross-hybridization by other PfEMP1 genes, we counted an element as switched on in a clinical sample only if its mean expression level exceeded its switched-off expression level eightfold or more.
Cloning and expression of PFD1140w.
cDNA encoding the predicted extracellular portion of PFD1140w (amino acid residues 34 through 347) was amplified using genomic 3D7 DNA and PCR with two primers: forward (CCCCTCGAGATGGACTACAAAGACGATGACGATAAGAAAAGTTCTAGTGGCTCATGTGTTAATAG) and reverse (CCCGGATCCTTAATTAGACAAAGCATCAATAATTTTTTTTTC). The forward primer introduced the coding sequence for the FLAG epitope. The PCR fragment was cloned into the XhoI and BamHI restriction sites of the pEU-E01-MCS vector (CellFree Sciences). Transcription and translation in the wheat germ cell-free system were performed according to the manufacturer's instructions. To confirm synthesis, synthetic protein was purified on anti-FLAG resin (Sigma) by using 3× FLAG peptide and analyzed by SDS gel electrophoresis and Coomassie blue staining (see Fig. 5A).
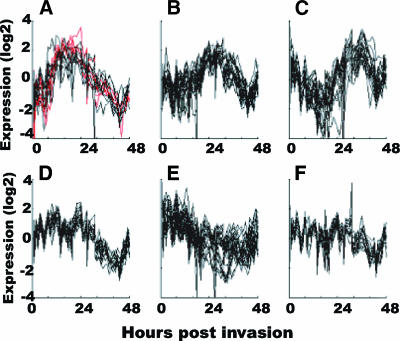
FIG. 5.
Coordinated expression of genes with increased expression in parasites from pregnant women. (A to F) In microarray data from reference 8, K means clustering was used to define six major expression profiles of genes during intraerythrocytic development of laboratory parasites. Four genes that are up-regulated in parasites from pregnant women (PFB0115w, PFD1140w, PFI1785w, and PFL0050c) (highlighted in red) cluster with a single group of genes that are most highly expressed during late-ring/early-trophozoite development.
Seroepidemiology of PFD1140w.
Mock and PFD1140w translation mixtures were diluted 1:10 with Superblock (Pierce) and incubated (100 μl per well) in anti-FLAG 96-well plates (Sigma) overnight at 4°C for rapid immobilization/purification of antigen. Human sera (from regions of malaria endemicity as well as from nonimmune individuals from the United States) were preincubated overnight at 4°C with equal volumes of 10 mg/ml normal mouse IgG and then diluted 1:100 and added to enzyme-linked-immunosorbent-assay plates. After 2 h of incubation at room temperature, wells were washed with phosphate-buffered saline-0.05% Tween 20 solution and incubated 1 h at room temperature with horseradish peroxidase-conjugated donkey anti-human IgG (heavy plus light chains) antibody (Jackson Immunoresearch) (diluted 1:1,000). Reactivity was measured using 100 μl/well of enhanced-chemiluminescence substrate (Amersham Biosciences) and a Fluoroskan Ascent FL fluorometer/luminometer (Thermo Labsystems). The value for the PFD1140-specific signal was calculated by subtracting the value for the control signal (for either the mock translation wells tested with the same serum or the PFD1140w wells tested with nonimmune serum, whichever was greater) and then normalized by dividing it by the value for the signal in the control wells (and as a consequence, the PFD1140w reactivity was expressed as the increase [n-fold] over the control value). Differences between groups were analyzed using the Mann-Whitney test.
RESULTS
Binding characteristics of clinical samples of parasites.
The receptor binding profiles of parasite samples were determined by standard static adhesion assays (16). Parasites obtained from placentas were assessed directly for their binding properties without prior in vitro cultivation. Placental IEs bound CSA but not other receptors, including HA (Table 2). Trophozoites derived in vitro from circulating peripheral parasites of both pregnant women and children were assayed for binding against the same receptors. Parasites cultured from maternal peripheral blood samples also adhered exclusively to CSA (Table 2). In contrast, parasites collected from children did not bind to CSA but instead adhered to a variety of receptors, including CD36, indicating the more diverse array of parasite forms infecting children (Table 2). No binding was detected for samples where parasitemia was low, consistent with other receptor binding studies (16).
TABLE 2.
Binding phenotypes of parasites from women with pregnancy malaria and from children with malaria
| Sample | Gravidity | Result for indicated receptor or test
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BSA | CD36 | CSA | HA | TSP | ICAM | M | Q-PCR | ||
| PLCNT 166a | Primigravid | − | − | + | − | − | − | + | + |
| PLCNT 221a | Primigravid | − | − | + | − | − | − | ND | + |
| PLCNT 222a | Primigravid | − | − | + | − | − | − | + | ND |
| PLCNT 918a | Primigravid | − | − | + | − | − | − | + | + |
| PLCNT 920a | Primigravid | − | − | + | − | − | − | ND | + |
| PLCNT 1010a | Primigravid | − | − | + | − | − | − | ND | ND |
| PLCNT 986a | Secundigravid | − | − | + | − | − | − | ND | ND |
| PLCNT 038a | Multigravid | − | − | + | − | − | − | + | ND |
| PLCNT 661a | Multigravid | − | − | + | − | − | − | + | + |
| PLCNT 836a | Multigravid | − | − | + | − | − | − | ND | + |
| CH-TR 095a | NA | − | + | − | − | − | + | + | |
| CH-TR 662a | NA | − | − | − | − | − | − | + | + |
| CH-TR 669a | NA | − | − | − | − | − | − | + | + |
| CH-TR 852a | NA | − | + | − | − | + | − | + | + |
| MT-PER 918b | Primigravid | − | − | + | − | − | − | + | ND |
| MT-PER 920b | Primigravid | − | − | − | − | − | − | + | + |
| MT-PER 1000b | Primigravid | − | − | − | − | − | − | ND | + |
| MT-PER 1010b | Primigravid | − | − | − | − | − | − | ND | + |
| MT-PER 986b | Secundigravid | − | − | + | − | − | − | ND | + |
| MT-PER 836b | Multigravid | − | − | + | − | − | − | + | + |
| CH-PER 073b | NA | + | + | ||||||
| CH-PER 135b | NA | − | + | − | − | − | − | + | + |
| CH-PER 140b | NA | − | − | − | − | + | + | + | ND |
| CH-PER 372b | NA | + | + | ||||||
| CH-PER 413b | NA | − | + | − | − | − | + | + | + |
| CH-PER 425b | NA | + | + | ||||||
| CH-PER 451b | NA | + | ND | ||||||
| CH-PER 711b | NA | − | + | − | − | + | − | + | + |
Binding phenotypes of placental parasites from women with pregnancy malaria and of trophozoites cultured from peripheral blood samples drawn from children with malaria. Parasites were extracted directly from infected placentas and tested for interaction with a panel of host receptors by using standard binding assays (16). + indicates ≥20 adherent infected red blood cells in 20 high-power fields for the control (BSA) and test (CD36, CSA, HA, TSP, and ICAM) receptors. Samples denoted with the PLCNT prefix are maternal placental parasites, and those with the CH-TR prefix are trophozoites cultured from infected children. ND, not done; NA, not applicable. Column M indicates samples that were used for microarrays. The transcriptional profiles of placental parasites and children's trophozoites were compared by microarray analysis and validated by Q-PCR. Additional samples were analyzed by Q-PCR for validation of microarray results. PLCNT 986 and PLCNT 1010 are placental samples from the same mothers as peripheral samples used only for Q-PCR validation.
Binding phenotype from peripheral parasites cultured to the trophozoite stage from women with pregnancy malaria and children with clinically diagnosed malaria. Samples denoted with the MT-PER prefix are from maternal peripheral parasites, and those with the CH-PER prefix are from peripheral parasites from children. As described in footnote a, the transcriptional profiles of peripheral parasites from women with pregnancy malaria and children with malaria were compared by microarray analysis and validated by Q-PCR.
Developmental stage of clinical samples defined by microarray analysis.
Genome-wide transcript expression profiles, taken at hourly intervals of the 48-h intraerythrocytic developmental cycle of HB3 parasites, have revealed a pattern of periodic expression for over 80% of the expressed genes (8, 39). The phaseogram depicting gene expression for the complete HB3 intraerythrocytic cycle (40) emphasizes that cell cycle timing is an important potential confounding factor in studies that examine phenotype-specific gene expression. We used whole-genome spotted microarrays to obtain the gene expression patterns of clinical parasite samples, studied immediately after collection or during the first cycle of in vitro development (Table 2). To map the cell cycle position for each sample, the expression profile obtained by microarray analysis was compared to the publicly available HB3 intraerythrocytic cycle time series (DeRisi laboratory malaria transcriptome database [http://malaria.ucsf.edu]). The HB3 time point with the closest resemblance to each clinical sample was identified using both Euclidean distance measurements and correlation comparisons between matched probes. The timing of all clinical samples is summarized in Table 3. The cell cycle position of each clinical sample calculated by the two methods was highly concordant, adding a measure of confidence to our analyses.
TABLE 3.
Summary of cell cycle positions of microarray samples as determined by Euclidean distance and correlation distance comparison with the 48-h asexual stage transcriptome of P. falciparum
| Clinical sample | Closest time point (h) for:
|
|
|---|---|---|
| Euclidean distance | Correlation distance | |
| MT-PER 836a | 13 | 13 |
| MT-PER 918a | 13 | 12 |
| MT-PER 920a | 13 | 13 |
| CH-PER 073a | 7 | 12 |
| CH-PER 135a | 10 | 10 |
| CH-PER 140a | 12 | 12 |
| CH-PER 372a | 16 | 13 |
| CH-PER 413a | 12 | 12 |
| CH-PER 425a | 13 | 13 |
| CH-PER 451a | 13 | 13 |
| CH-PER 711a | 12 | 10 |
| PLCNT 038b | 31 | 31 |
| PLCNT 166b | 24 | 24 |
| PLCNT 222b | 31 | 31 |
| PLCNT 661b | 24 | 24 |
| PLCNT 918b | 31 | 31 |
| CH-TR 095b | 31 | 31 |
| CH-TR 662b | 47 | 47 |
| CH-TR 669b | 31 | 31 |
| CH-TR 852b | 31 | 31 |
Cell cycle timing of peripheral parasites isolated from women with pregnancy malaria and children with malaria that were used to identify genes that are differentially expressed during the ring stage of development (the stage immediately preceding parasite adhesion to the placenta). The HB3 P. falciparum transcriptome database (DeRisi laboratory) was used to map the time after invasion of red blood cells for all clinical samples used for microarray analysis.
Cell cycle timing of placental parasites and trophozoites cultured from children used for comparison of gene expression levels for placental binding and nonbinding parasites.
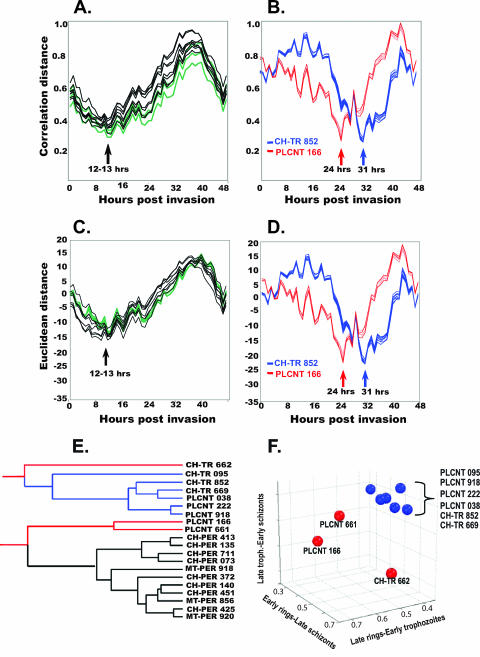
These measurements indicate that the peripheral samples are well matched with respect to cell cycle timing, minimizing this as a major confounder in our analysis of binding phenotype differences between groups. The closest correlation distance for all peripheral samples maps to between 10 and 13 h of the HB3 blood stage cycle, with the majority of samples falling between 12 and 13 h (Fig. 1A). The corresponding overlaid Euclidean distance plots for all peripheral samples show the same trends (Fig. 1C). Correlation distance plots for replicates of all samples are also shown in Fig. S1 in the supplemental material. All Euclidean distance plots are shown in Fig. S2 in the supplemental material. A high level of reproducibility between arrays is seen.
FIG. 1.
Timing analysis of each clinical sample in the 48-h intraerythrocytic cycle. (A) Correlation distance plots comparing averages for replicates for each peripheral blood parasite sample with the 51 time points from the 48-h HB3 transcriptional profile analysis (8). The three peripheral samples from women with pregnancy malaria are shown in green, and eight peripheral samples from children with malaria are shown in black. (B) Correlation distance plot comparing averages for replicate samples of placental parasite PLCNT 166 and averages for replicates of CH TR 852. (C and D) Euclidean distance plots for samples in panels A and B, respectively. (E). Hierarchical clustering of correlation time profiles of all clinical samples. (F). Placental trophozoite samples and cultured trophozoite samples from children were loaded onto three principal-component coordinates to compare their positions in the cell cycle. Child-derived trophozoite samples CH-TR 095, CH-TR 852, and CH-TR 669 and placental samples PLCNT 038, PLCNT 222, and PLCNT 918 are comparably positioned (shown in blue). Placental samples PLCNT166 and PLCNT 661 have greater representation in the late ring-early trophozoite component (shown in red). Sample CH-TR 662 (in red) has a distinct timing profile. The 31-h cluster is shown in blue. Samples are identified left to right.
Placental parasite samples and trophozoites cultured from child-derived samples show greater diversity in cell cycle timing than the peripheral parasite samples. The child-derived sample CH-TR 662 is poorly synchronized and most nearly resembles synchronized HB3 parasites at the 47-h time point rather than those at the trophozoite stage (between 18 and 32 h) (see Fig. S1 and S2 in the supplemental material). This sample was excluded from all further analyses. Two out of five placental parasite samples map to 24 h, while all other placental samples, as well as all of the remaining trophozoite samples from children, most closely resemble the 31-h HB3 profile. To visualize these differences, child-derived sample CH-TR 852 with a best fit at 31 h is shown superimposed on the PLCNT 166 correlation plot (Fig. 1B) and the corresponding Euclidean distance plot (Fig. 1D), which maps to 24 h. Correlation and Euclidean distance measurements for the placental and child-derived trophozoite samples indicate that the two methods yield similar results (see Fig. S1 and S2 in the supplemental material). Hierarchical clustering of all time profiles places PLCNT 661 and PLCNT 166 between the ring and trophozoite samples (Fig. 1E). The correlation and Euclidian distance results were consistent with the PCA results in which the 48-h intraerythrocytic developmental cycle is reduced to three principal components corresponding to the early ring-late schizont, late ring-early trophozoite, and late trophozoite-early schizont stages (Fig. 1F).
var2csa is highly expressed by peripheral parasites from pregnant women.
We aligned 3D7, IT4, and Dd2 sequences of var2csa and identified nine unique, well conserved 70-mer sequences for use as microarray elements (Fig. 2). These elements are also generally conserved in the recently sequenced Ghanaian clinical parasite sample (Fig. 2). Multiple pairwise comparisons between three maternal peripheral samples and eight samples from children were performed (see Table S1 in the supplemental material). Four out of seven var2csa elements were well detected by a probe prepared from maternal parasites; two of three elements that performed poorly were the least conserved compared to the Ghanaian sequence. Two additional elements with greater than 90% sequence identity with var2csa were discovered during analysis of the hybridization experiments. Element var2csa 4038 (derived from a cDNA clone of the placental parasite pl28b) was originally thought to be a divergent DBLγ domain of var1csa (19) but is identified here as a clinical variant of var2csa. The other element maps to the probable 5′ untranslated region (37) of var2csa 653 bp upstream from the putative initiator methionine (Fig. 2).
FIG. 2.
var2csa microarray elements. Schematic of consensus 70-mer nucleotide microarray elements showing DBL domain assignments. Elements were designed by aligning var2csa sequences from different laboratory strains of P. falciparum. 3D7, Ghana-1, and Ghana-2 indicate percent sequence identity of array elements with 3D7 and two recently sequenced var2csa homologs obtained from a clinical sample (Ghanaian P. falciparum genome sequence; Sanger Center). Operon element M59463_1 (made against MAL13P1.353, no longer annotated) maps to bp −653 relative to the PFL0030c translation start site. The element at position 4038 was designed from a DNA sequence amplified from placental isolate pl 28b, using degenerate DBLγ domain primers (19). Element positions are relative to the 3D7 gene PFL0030c. UTR, untranslated region.
Of 396 possible var elements spotted on these arrays, the probe prepared from pregnancy malaria peripheral parasites hybridized exclusively to 11 var2csa elements (Fig. 3A). In contrast, peripheral parasites from children showed no unifying trends. Signals were reliably detected for 56 elements that have homology to 30 genes and pseudogenes in the 3D7 genome. Elements designed from conserved regions of group A PfEMP1 genes were most frequently recognized by parasites from children, and some minor trends can be observed. For example, four PFD1235w elements, positioned along 1,850 bp of the gene, were recognized by CH-PER 711, consistent with a high degree of conservation. Signals from three out of four elements of PFF0020c were also detected for CH-PER 711. In seven other cases, two elements from the same 3D7 gene hybridized with the same clinical sample. Interestingly, there is very little consistent recognition of a particular element across samples from children (Fig. 3B). The diversity of var transcripts detected in parasites from children could be due in part to differences in binding phenotype profiles between parasites. Alternatively, CD36 binding is common to most of these samples from children, and multiple 3D7 var genes appear to have the capacity to bind CD36 in vitro (55), which may also be the case in vivo.
FIG. 3.
PfEMP1 gene expression. (A) Checkerboard representation of expression by members of the PfEMP1 gene family by different clinical samples. Gray boxes denote samples that hybridized to that PfEMP1 probe. PfEMP1 genes were grouped according to the classification scheme in reference 37. The probe position for each PfEMP1 element is based on the 3D7 nucleotide sequence and is indicated as part of each gene name. (B, C, D) Comparison of signal intensities (filled bars) among clinical samples for elements PFL0030c-5053 (B), PFC0005w-4450 (C), and PFL1955w-5979 (D). Open bars show background intensity.
Six up-regulated genes distinguish pregnancy malaria parasites from child-derived parasites at both the ring and trophozoite stages.
Multiple regression analysis of all sample replicates was performed, and an average value was extracted for each element. Hierarchical clustering of the Euclidean averaged distances produced a distinct cluster that included multiple var2csa elements and elements from eight genes (Fig. 4A). All were expressed eightfold or higher in maternal peripheral parasites compared to what was found in peripheral parasites from children. PFB0115w was expressed 47.5-fold higher in maternal parasites, a magnitude of differential expression comparable to that of the best-recognized elements of var2csa. Q-PCR of peripheral parasite samples from women with pregnancy malaria (including some samples that were and some that were not used for microarray studies) confirmed that these genes were significantly more highly expressed by parasites from maternal peripheral blood samples (Fig. 4B).
FIG. 4.
Genes with increased expression in parasites obtained from women with pregnancy malaria. (A) Heat map of highly up-regulated genes expressed by peripheral parasites from women with pregnancy malaria based on Euclidean hierarchical clustering. (B) Expression levels of up-regulated genes identified by microarray analysis compared to seryl tRNA synthase constitutive control levels as detected by Q-PCR. (C) Heat map of transcription by trophozoite stage parasites, focused on the var2csa gene cluster identified in maternal peripheral parasites. (D) Q-PCR analysis of trophozoite stage gene expression.
As was seen for the peripheral parasite microarrays (see Table S1 in the supplemental material), the number of genes that were expressed twofold or higher by placental parasites is small (see Table S2 in the supplemental material). Among the genes that were upregulated in peripheral parasites of pregnant women, the expression levels of PFB0115w, PFD1140w, PFI1785w, PFL0050c, MAL13P1.320, and var2csa were also upregulated in placental parasites, whereas those of MAL13P1.470, PFF0435w, and PFL1385c were not (Fig. 4C). Genes found by microarray analysis to have increased expression in placental parasites were validated by Q-PCR (Fig. 4D).
In total, 8 different maternal samples derived from either peripheral blood samples or placentas from women with pregnancy malaria were compared with 11 parasite samples collected from peripheral blood samples from children and assayed directly or cultured to the trophozoite stage. A small number of genes were found to be expressed at higher levels by parasites from women with pregnancy malaria. Despite differences in the developmental stages of maternal parasites, six genes were common to both analyses. In addition to these six genes, MAL7P1.225 and PF10_0013 were more highly expressed by placental parasites, while MAL13P1.470 was more highly expressed by ring stage parasites from pregnant women.
Genes up-regulated in pregnancy malaria parasites have coordinated timing of expression.
The transcription profiles for genes that differ most consistently over the course of blood stage development were extracted from the intraerythrocytic cycle database (malaria transcriptome database [http://malaria.ucsf.edu]). K means clustering produced six expression profiles (Fig. 5). Four of six clusters have well-defined expression patterns roughly corresponding to peaks at the ring stage, late ring/early trophozoite stage, mid- to late trophozoite stage, and schizont/early ring stage (Fig. 5A, B, C, and D). One cluster is less well defined and has broader transcript timing (Fig. 5E), and one cluster is composed of genes whose expression levels are close to the background level across the intraerythrocytic cycle (Fig. 5F).
The genes (PFB0115w, PFD1140w, PFI1785w, and PFL0050c) that were most highly expressed in both peripheral and placental parasites from pregnant women cluster with genes expressed maximally at the late ring/early trophozoite stage (Fig. 5A). This expression pattern coincides with the disappearance of trophozoite stage parasites from the peripheral circulation because of sequestration in tissues. Expression of var2csa was not detected in the HB3 time series; maximal var2csa expression was observed at 6 hours postinfection in the 3D7 blood stage time course (malaria transcriptome database [http://malaria.ucsf.edu]). Other studies indicate that the var2csa message is present during the ring and early trophozoite stages (60). MAL13P1.320 does not follow the same transcription profile and instead clusters with genes expressed at a low level across much of the HB3 cell cycle (Fig. 5F).
Serum recognition by PFD1140w is gender and parity specific.
Genes with increased transcription in parasites from pregnant women were expressed by in vitro translation and tested by an enzyme-linked immunosorbent assay for reactivity against East African sera. Thirty-three sera from multigravid and 20 sera from primigravid women from a high-malaria-transmission area in Tanzania were compared for reactivity against PFD1140w, PFI1785w, PFB0115w, MAL13P1.320, and MAL13P1.470. Reactivity against recombinant PFD1140w was significantly higher with sera from multigravid women, suggesting that antibodies are acquired over successive pregnancies (Fig. 6). None of the other expressed proteins showed significant parity-specific seroreactivity profiles. PFB0115w was more reactive against sera from multigravid women, but differences between multigravid and primigravid sera were not statistically significant. Other expressed proteins were poorly recognized by sera, possibly because of problems with protein folding (data not shown).
FIG. 6.
PFD1140w seroreactivity. Recombinant PFD1140w is recognized by human sera from areas of malaria endemicity in a gender- and parity-dependent manner. (A) Coomassie-stained gel of affinity-purified cell-free expressed PFD1140w. Control, material purified from mock-translated reaction mixture. M, molecular mass markers with corresponding values (in kilodaltons). Low-molecular-mass material in both samples is FLAG peptide used for elution. (B) Reactivity of PFD1140w with sera from Muheza, Tanzania, where malaria transmission is intense. Multi, sera from multigravid women. Primi, sera from primigravid women. AU, arbitrary units. P, Mann-Whitney P value. N indicates the number of samples. The top of the box, bottom of the box, and line through the middle of the box correspond to the 75th, 25th, and 50th (median) percentiles, respectively. The whiskers indicate the 10th and 90th percentiles. (C) Reactivity of PFD1140w with sera from Kenya, where malaria transmission is intense. Male, sera from male donors. Other abbreviations and representations are as described for panel B.
In rural areas around towns such as Muheza, Tanzania, most women have their first pregnancy within a short age window, and therefore it is generally difficult to age match primigravidae with multigravidae. To eliminate age as a confounder, we compared the reactivities of sera from 22 multigravid women (median age, 28 years) with those of sera from 22 males (median age, 29 years) living in an area of intense malaria transmission near Kisumu, Kenya. Sera from multigravid women were significantly more reactive than male sera (Fig. 6), indicating that differences in reactivity were not related to age but were related to pregnancy and parity. The seroreactivity profile shown for PFD1140w matches that previously reported for VAR2CSA using the same sera (50). Reactivity to the control malaria antigen apical membrane antigen (AMA-1) did not differ significantly between primigravidae and multigravidae in Tanzania or between males and multigravidae in Kenya, and all East African sera reacted to this antigen in our studies (50).
DISCUSSION
The receptor binding profiles of parasites derived from pregnant women did not overlap with those for children (Table 2), thus providing an opportunity to identify differentially regulated genes that distinguish placental parasites from other parasite forms.
Since var gene transcription is greatest during ring stage development (36), we speculated that other adhesion proteins or proteins related to the binding phenotype might have coordinated expression in ring stage parasites. We assumed that peripheral parasites were representative of placental parasites, because peripheral parasites of pregnant women mature into CSA-binding trophozoites (16, 17) and develop seroreactivity profiles similar to those of placental parasites (49). Consistent with this, we found a striking overlap in up-regulated genes from both peripheral and placental parasites (Table 4).
TABLE 4.
Characteristics of genes with increased expression in pregnancy malaria parasites by microarray analysis
| Gene | Fold increase in expression of:
|
Result for:
|
Potential export sequencee | Mass (kDa) | Maximum expression time (h)f | Stage(s) of expressiong | No. of paralogs | Function | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal peripheral parasites | Placental parasites | SS/TMa | VTS | PEXEL | |||||||
| PFL0030c | 14.2 | 6.4 | −/+b | + | + | RWLTE | 355.2 | ND | ND | 59 | PfEMP1 |
| PFB0115w | 47.5 | 4.0 | −/−c | − | − | 141.8 | 16 | ET,LT | 1 | Hypothetical | |
| PFD1140w | 16.9 | 6.3 | +/+b | − | + | RNLSE | 40.8 | 18 | LR,ET,LT | 3 | Hypothetical |
| MAL13P1.320 | 16.9 | 3.4 | +/+ | − | − | 80.1 | ND | LR,ET,LT | 1 | Hypothetical | |
| PFL0050c | 9.6 | 6.2 | −/−c | + | − | RILSS | 77.1 | 18 | ES | 1 | Hypothetical |
| PFI1785W | 8.2 | 10.3 | −/−c | + | − | RISLE | 39.5 | 22 | ET,LT | 17 | Hypothetical |
| PF10_0013 | NI | 4.4 | +/− | − | + | RLISE | 27.6 | 18 | LR | 4 | Hypothetical |
| MAL13P1.470 | 9.9 | NI | −/+b | − | − | RNLSE | 48.9 | 20 | NP | 42 | Hypothetical |
| MAL7P1.225 | 0.6 | 5.1 | −/+b,d | − | − | RNLSE | 29.9 | 18 | NP | 42 | Hypothetical |
The transmembrane domain (TM) was predicted by transmembrane prediction alogrithm TMHMM unless otherwise stated. SS, signal sequence.
Potential signal anchor sequence.
TM was predicted by alternate transmembrane prediction algorithms TMAP, TMPRED, and/or TOPPRED2.
Alternate annotation, chr 7 glimmerm_35.
Predicted by PlasmoDB 5.2.
For a synchronized HB3 time series profile (8).
3D7 expression time course (38). LR, late ring; ET, early trophozoite; LT, late trophozoite; ES, early schizont; NP, not probed; ND, not detected; NI, not increased.
Cell cycle analysis.
With the exception of child-derived trophozoite parasites that had been grown in culture overnight, all clinical samples used in these studies were processed immediately after collection for subsequent use in transcriptional studies. To determine the cell cycle timing for each clinical sample used in this study, the transcriptional profile obtained by microarray analysis was compared with the transcriptional profile at every hour of the 48-h intraerythrocytic cycle, using the publicly available data set (malaria transcriptome database [http://malaria.ucsf.edu]), by Euclidean and correlation distance measurements. Similar results were obtained using both methods (Fig. 1). We found that ring stage parasites from peripheral blood samples matched HB3 timing most closely at 10 to 13 h after invasion of the red blood cell. The basis for the striking concordance in cell cycle timing of peripheral parasites is unknown but may be due in part to the increased level of transcription in late-ring-stage (10 to 13 h) parasites, which could contribute disproportionately to the hybridization signal. Regardless, the closely matched cell cycle timing gives us greater confidence that expression differences between these parasite phenotypes are related to CSA binding or to the specialized physiological milieu of the placenta rather than to differences in the cell cycle.
In contrast to the peripheral parasites analyzed here, the trophozoite stage samples fall into two distinct time classes at 24 h and 31 h of development. When the 31-h placental samples (PLCNT 038, PLCNT 222, and PLCNT 918) are compared to 31-h child-derived trophozoite samples (CH-TR 095 and CH-TR 852), a subset of the genes identified in the ring stage comparisons (PFD1140w, PFB0115w, PFI1785w, PFL0050c and PFL0030c/var2csa) are found to be expressed at two- to fourfold-higher levels in placental parasites (see Table S2 in the supplemental material). Comparison of the 24-h placental parasite samples (PLCNT 661 and PLCNT 166) with the 31-h trophozoites from children suggests a much higher level of differential expression in these genes (see Table S2 in the supplemental material), presumably due in part to the differences in cell cycle timing. This emphasizes the importance of determining the developmental stage of parasites in clinical samples.
var2csa is up-regulated in all parasite samples from women with pregnancy malaria.
VAR2CSA is a semiconserved member of the PfEMP1 family (34, 60) that has been previously implicated as a mediator of adhesion to the placental receptor CSA. We found that var2csa was differentially expressed by both peripheral and placental binding parasites of pregnant women. In contrast, var2csa expression was reliably detected in only one child-derived sample (CH-PER 413), and here, only a few elements were hybridized and the signal was much less intense than those in maternal samples (Fig. 3B). The var2csa hybridization pattern of CH-PER 413 may have resulted from cross-hybridization with another expressed PfEMP1; however, this was not seen for other samples from children. Alternatively, the sequence of var2csa expressed by this parasite may diverge extensively from the sequence of var2csa expressed by parasites from pregnant women. Curiously, two elements recognized by the CH-PER 413 probe appear to be the most variant of those designed from conserved regions of this gene. This may indicate that a subset of var2csa variants is specifically associated with placental parasites.
Five genes are coexpressed with var2csa by parasites causing pregnancy malaria.
We identified a small cohort of genes that mirror the var2csa expression profile in both peripheral and placental parasites from pregnant women. Genes included in this group predict proteins with transmembrane and/or signal sequences (Table 4). Three of the five differentially expressed genes identified here have putative export motifs (Table 4) that are consistent with transit to the erythrocyte (30, 44). PFD1140w has a PEXEL sequence (44), while PFL0050c and PFI1785w each have a VTS motif (30). Interestingly, PFI1785w belongs to a P. falciparum family composed of 16 uncharacterized hypothetical genes, most of which have predicted export sequences (PlasmoDB). PFB0115w and MAL13P1.320 have neither a PEXEL nor a VTS motif. However, this does not exclude trafficking of these proteins to the red blood cell by alternate export pathways, as has been suggested for skeleton binding protein 1 (sbp1 protein) and Maurer's cleft histidine-rich protein, which lack PEXEL/VTS motifs but nevertheless localize to the Maurer's clefts in the red blood cell cytoplasm.
Additional genes were differentially expressed by only ring stage or only mature-stage parasites from pregnant women and may play a role in the placental parasite phenotype as well. These genes include two members of the HISTa gene family (61), consisting of 42 paralogs in P. falciparum (P. falciparum gene database, Sanger Center [http://www.genedb.org/]) that encode proteins with putative PEXEL sequences (61) as well as PF10_0013, which has a PEXEL motif (44) and has been localized to the Maurer's cleft by proteomic analysis (68) (Table 4). Future studies will be required to determine whether these proteins are trafficked to the erythrocyte as well as how these expanded gene families affect the pathogenesis of P. falciparum malaria. A recent photolabeling study suggested that a 22-kDa protein on the IE surface may bind CSA (26). None of the genes identified here predict a protein of this size, although PF10_0013 (27.6 kDa) and MAL7P1.225 (29.9 kDa) predict slightly larger molecules.
Genes identified in the placental malaria cohort have been linked to adhesion phenotypes in two other microarray studies. Mok and coworkers found var2csa to be one of three var genes expressed at higher levels by rosetting parasites than by CD36-selected 3D7 parasites (45). This is curious because rosetting parasites are common in children, where var2csa transcription is atypical, but rarely observed in placental isolates, where var2csa transcription is generally detected (14, 56). Furthermore, the parasites selected to form rosettes failed to bind CSA (although they did bind to placental sections in a CSA-independent manner). Notably, PFB0115w was also differentially expressed by the rosetting parasites, suggesting that expression of var2csa and PFB0115w may be linked, as was seen in our study.
An earlier study used microarrays to compare FCR3 parasites selected to bind either CSA or CD36 (52). The microarrays included FCR3 var-specific probes to allow detection of PfEMP1 genes. CD36-selected cells differentially expressed a number of var genes, while only var2csa was differentially expressed by CSA-selected parasites. Many non-var genes were also found to have increased expression by FCR3 CSA-binding parasites, including PFB0115w, PFD1140w, and MAL13P1.470, which were among the most highly differentially expressed genes at both the ring and trophozoite stages (52). In contrast to the FCR3 study, we found only six genes with increased expression in all maternal parasite samples. The smaller cohort generated by our study might result because more samples were compared (8 maternal samples were compared to 11 samples from children) or because our studies used clinical samples that may better discriminate relevant genes. In the FCR3 study, two biological replicates were analyzed at the ring and trophozoite stages. The overlap in gene expression between parasites from women with pregnancy malaria and CSA-selected FCR3 parasites suggests that the genes identified here as belonging to the pregnancy malaria cohort merit further investigation. Our results suggest that only a few differentially expressed genes, including var2csa, distinguish placental parasites. However, due to sequence variation, we cannot exclude roles for highly polymorphic genes, like rifins, stevors, Pfmc-2TM, and other PfEMP1 genes, in the development of the placental parasite phenotype (9).
Seroreactivity of PFD1140w is gender and parity specific.
VAR2CSA has been previously shown to have gender- and parity-specific recognition by immune serum that corresponds to the pattern of acquired immunity to pregnancy malaria (59, 67). PFD1140w showed a similar seroreactivity profile (Fig. 6). Whether antibodies against PFD1140w impair or block adhesion to CSA or have some other functional activity against placental parasites will be addressed in future studies. PFB0115w had higher reactivity with multigravid sera than with male sera, but these differences were not significant. PFB0115w should be studied in larger seroepidemiology surveys to assess whether this trend reflects a real difference in seroreactivity.
In conclusion, our results indicate that a small suite of genes is associated with the development of the placental parasite phenotype. Although the roles of the corresponding proteins in malaria pathogenesis or immunity remain unclear, we speculate that some of these proteins may have functions to interface with the pregnant host in the specialized milieu of the placenta, such as the putative role in which VAR2CSA adheres to CSA. Any proteins that localize to the surface of the IE will become important candidates for a pregnancy malaria vaccine, particularly in view of their highly conserved sequences. Defining the separate roles of these genes in the development of placental parasites could lead to a better understanding of mechanisms by which parasites acquire distinct binding properties and yield novel targets for intervention.
Supplementary Material
Acknowledgments
We thank Chris Ramsborg for critical reading of the manuscript; Wonjong Moon, Atis Muehlenbachs, Jay Gerlach, and Paul Shannon for help with data analysis; Jeff Dorfman for helpful discussions; Lori Anderson for technical assistance; Sue Kraemer, Amy Springer, and Joe Smith for providing advice and sharing unpublished data; JinLong Li for IT support; Maximillian Mpina, Martin Mhando, and Dalley Andeoli for performing binding assays; and the MOMS Project nurses stationed at Muheza DDH who collected and processed the samples used in this study.
This work was supported by the U.S. National Institutes of Health (grant AI52059), the Bill and Melinda Gates Foundation (grant 29202), and the Grand Challenges in Global Health/Foundation for the NIH (grant 1364).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 13 August 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Achur, R. N., M. Valiyaveettil, and D. C. Gowda. 2003. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. J. Biol. Chem. 278:11705-11713. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, K. T., L. A. Pirrit, J. M. Przyborski, C. P. Sanchez, Y. Sterkers, S. Ricken, H. Wickert, C. Lepolard, M. Avril, A. Scherf, J. Gysin, and M. Lanzer. 2003. Recovery of adhesion to chondroitin-4-sulphate in Plasmodium falciparum varCSA disruption mutants by antigenically similar PfEMP1 variants. Mol. Microbiol. 49:655-669. [DOI] [PubMed] [Google Scholar]
- 4.Barfod, L., N. L. Bernasconi, M. Dahlback, D. Jarrossay, P. H. Andersen, A. Salanti, M. F. Ofori, L. Turner, M. Resende, M. A. Nielsen, T. G. Theander, F. Sallusto, A. Lanzavecchia, and L. Hviid. 2007. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol. Microbiol. 63:335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baruch, D. I., J. A. Gormely, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botha, M., E. R. Pesce, and G. L. Blatch. 2007. The Hsp40 proteins of Plasmodium falciparum and other apicomplexa: regulating chaperone power in the parasite and the host. Int. J. Biochem. Cell Biol. [Epub ahead of print.] doi: 10.1016/j.biocel.2007.02.011. [DOI] [PubMed]
- 8.Bozdech, Z., M. Llinas, B. L. Pulliam, E. D. Wong, J. Zhu, and J. L. DeRisi. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdech, Z., J. Zhu, M. P. Joachimiak, F. E. Cohen, B. Pulliam, and J. L. DeRisi. 2003. Expression profiling of the schizont and trophozoite stages of Plasmodium falciparum with a long-oligonucleotide microarray. Genome Biol. 4:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffet, P. A., B. Gamain, C. Scheidig, D. Baruch, J. D. Smith, R. Hernandez-Rivas, B. Pouvelle, S. Oishi, N. Fujii, T. Fusai, D. Parzy, L. H. Miller, J. Gysin, and A. Scherf. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA 96:12743-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke, B. M., D. W. Buckingham, F. K. Glenister, K. M. Fernandez, L. H. Bannister, M. Marti, N. Mohandas, and R. L. Coppel. 2006. A Maurer's cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J. Cell Biol. 172:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy, M. F., T. J. Byrne, S. R. Elliott, D. W. Wilson, S. J. Rogerson, J. G. Beeson, R. Noviyanti, and G. V. Brown. 2005. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol. Microbiol. 56:774-788. [DOI] [PubMed] [Google Scholar]
- 14.Duffy, M. F., A. Caragounis, R. Noviyanti, H. M. Kyriacou, E. K. Choong, K. Boysen, J. Healer, J. A. Rowe, M. E. Molyneux, G. V. Brown, and S. J. Rogerson. 2006. Transcribed var genes associated with placental malaria in Malawian women. Infect. Immun. 74:4875-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott, S. R., M. F. Duffy, T. J. Byrne, J. G. Beeson, E. J. Mann, D. W. Wilson, S. J. Rogerson, and G. V. Brown. 2005. Cross-reactive surface epitopes on chondroitin sulfate A-adherent Plasmodium falciparum-infected erythrocytes are associated with transcription of var2csa. Infect. Immun. 73:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried, M., G. J. Domingo, C. D. Gowda, T. K. Mutabingwa, and P. E. Duffy. 2006. Plasmodium falciparum: chondroitin sulfate A is the major receptor for adhesion of parasitized erythrocytes in the placenta. Exp. Parasitol. 113:36-42. [DOI] [PubMed] [Google Scholar]
- 17.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 18.Fried, M., and P. E. Duffy. 1998. Maternal malaria and parasite adhesion. J. Mol. Med. 76:162-171. [DOI] [PubMed] [Google Scholar]
- 19.Fried, M., and P. E. Duffy. 2002. Two DBLgamma subtypes are commonly expressed by placental isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 122:201-210. [DOI] [PubMed] [Google Scholar]
- 20.Fried, M., R. M. Lauder, and P. E. Duffy. 2000. Plasmodium falciparum: adhesion of placental isolates modulated by the sulfation characteristics of the glycosaminoglycan receptor. Exp. Parasitol. 95:75-78. [DOI] [PubMed] [Google Scholar]
- 21.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 22.Gamain, B., S. Gratepanche, L. H. Miller, and D. I. Baruch. 2002. Molecular basis for the dichotomy in Plasmodium falciparum adhesion to CD36 and chondroitin sulfate A. Proc. Natl. Acad. Sci. USA 99:10020-10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamain, B., A. R. Trimnell, C. Scheidig, A. Scherf, L. H. Miller, and J. D. Smith. 2005. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J Infect. Dis. 191:1010-1013. [DOI] [PubMed] [Google Scholar]
- 24.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerlitz, B., T. Hassell, C. J. Vlahos, J. F. Parkinson, N. U. Bang, and B. W. Grinnell. 1993. Identification of the predominant glycosaminoglycan-attachment site in soluble recombinant human thrombomodulin: potential regulation of functionality by glycosyltransferase competition for serine474. Biochem. J. 295:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gowda, A. S., S. V. Madhunapantula, R. N. Achur, M. Valiyaveettil, B. P. Veer, and D. C. Gowda. 2007. Structural basis for the adherence of Plasmodium falciparum infected erythrocytes to chondroitin 4-sulfate and design of novel photoactivable reagents for the identification of parasite adhesive proteins. J. Biol. Chem. 282:916-928. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt, H. L., and R. W. Snow. 2004. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin. Microbiol. Rev. 17:760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gysin, J., B. Pouvelle, N. Fievet, A. Scherf, and C. Lepolard. 1999. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect. Immun. 67:6596-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haldar, K., and N. Mohandas. 2007. Erythrocyte remodeling by malaria parasites. Curr. Opin. Hematol. 14:203-209. [DOI] [PubMed] [Google Scholar]
- 30.Hiller, N. L., S. Bhattacharjee, C. van Ooij, K. Liolios, T. Harrison, C. Lopez-Estrano, and K. Haldar. 2004. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306:1934-1937. [DOI] [PubMed] [Google Scholar]
- 31.Jensen, A. T., H. D. Zornig, C. Buhmann, A. Salanti, K. A. Koram, E. M. Riley, T. G. Theander, L. Hviid, and T. Staalsoe. 2003. Lack of gender-specific antibody recognition of products from domains of a var gene implicated in pregnancy-associated Plasmodium falciparum malaria. Infect. Immun. 71:4193-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knuepfer, E., M. Rug, N. Klonis, L. Tilley, and A. F. Cowman. 2005. Trafficking of the major virulence factor to the surface of transfected P. falciparum-infected erythrocytes. Blood 105:4078-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraemer, S. M., S. A. Kyes, G. Aggarwal, A. L. Springer, S. O. Nelson, Z. Christodoulou, L. M. Smith, W. Wang, E. Levin, C. I. Newbold, P. J. Myler, and J. D. Smith. 2007. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates. BMC Genomics 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraemer, S. M., and J. D. Smith. 2003. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 50:1527-1538. [DOI] [PubMed] [Google Scholar]
- 35.Kriek, N., L. Tilley, P. Horrocks, R. Pinches, B. C. Elford, D. J. Ferguson, K. Lingelbach, and C. I. Newbold. 2003. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol. Microbiol. 50:1215-1227. [DOI] [PubMed] [Google Scholar]
- 36.Kyes, S., R. Pinches, and C. Newbold. 2000. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105:311-315. [DOI] [PubMed] [Google Scholar]
- 37.Lavstsen, T., A. Salanti, A. T. Jensen, D. E. Arnot, and T. G. Theander. 2003. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Roch, K. G., J. R. Johnson, L. Florens, Y. Zhou, A. Santrosyan, M. Grainger, S. F. Yan, K. C. Williamson, A. A. Holder, D. J. Carucci, J. R. Yates III, and E. A. Winzeler. 2004. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 14:2308-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llinas, M., and H. A. del Portillo. 2005. Mining the malaria transcriptome. Trends Parasitol. 21:350-352. [DOI] [PubMed] [Google Scholar]
- 40.Llinas, M., and J. L. DeRisi. 2004. Pernicious plans revealed: Plasmodium falciparum genome wide expression analysis. Curr. Opin. Microbiol. 7:382-387. [DOI] [PubMed] [Google Scholar]
- 41.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 42.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 43.Marti, M., J. Baum, M. Rug, L. Tilley, and A. F. Cowman. 2005. Signal-mediated export of proteins from the malaria parasite to the host erythrocyte. J. Cell Biol. 171:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marti, M., R. T. Good, M. Rug, E. Knuepfer, and A. F. Cowman. 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306:1930-1933. [DOI] [PubMed] [Google Scholar]
- 45.Mok, B. W., U. Ribacke, G. Winter, B. H. Yip, C. S. Tan, V. Fernandez, Q. Chen, P. Nilsson, and M. Wahlgren. 2007. Comparative transcriptional analysis of isogenic Plasmodium falciparum clones of distinct antigenic and adhesive phenotypes. Mol. Biochem. Parasitol. 151:184-192. [DOI] [PubMed] [Google Scholar]
- 46.Mutabingwa, T. K., M. C. Bolla, J. L. Li, G. J. Domingo, X. Li, M. Fried, and P. E. Duffy. 2005. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthusamy, A., R. N. Achur, V. P. Bhavanandan, G. G. Fouda, D. W. Taylor, and D. C. Gowda. 2004. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am. J. Pathol. 164:2013-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunes, M. C., J. P. Goldring, C. Doerig, and A. Scherf. 2007. A novel protein kinase family in Plasmodium falciparum is differentially transcribed and secreted to various cellular compartments of the host cell. Mol. Microbiol. 63:391-403. [DOI] [PubMed] [Google Scholar]
- 49.Ofori, M. F., T. Staalsoe, V. Bam, M. Lundquist, K. P. David, E. N. Browne, B. D. Akanmori, and L. Hviid. 2003. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect. Immun. 71:1584-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oleinikov, A. V., E. Rossnagle, S. Francis, T. K. Mutabingwa, M. Fried, and P. E. Duffy. 2007. Effects of sex, parity, and sequence variation on seroreactivity to candidate pregnancy malaria vaccine antigens. J. Infect. Dis. 196:155-164. [DOI] [PubMed] [Google Scholar]
- 51.Papakrivos, J., C. I. Newbold, and K. Lingelbach. 2005. A potential novel mechanism for the insertion of a membrane protein revealed by a biochemical analysis of the Plasmodium falciparum cytoadherence molecule PfEMP-1. Mol. Microbiol. 55:1272-1284. [DOI] [PubMed] [Google Scholar]
- 52.Ralph, S. A., E. Bischoff, D. Mattei, O. Sismeiro, M. A. Dillies, G. Guigon, J. Y. Coppee, P. H. David, and A. Scherf. 2005. Transcriptome analysis of antigenic variation in Plasmodium falciparum-var silencing is not dependent on antisense RNA. Genome Biol. 6:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeder, J. C., A. F. Cowman, K. M. Davern, J. G. Beeson, J. K. Thompson, S. J. Rogerson, and G. V. Brown. 1999. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl. Acad. Sci. USA 96:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 55.Robinson, B. A., T. L. Welch, and J. D. Smith. 2003. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 47:1265-1278. [DOI] [PubMed] [Google Scholar]
- 56.Rogerson, S. J., J. G. Beeson, C. G. Mhango, F. K. Dzinjalamala, and M. E. Molyneux. 2000. Plasmodium falciparum rosette formation is uncommon in isolates from pregnant women. Infect. Immun. 68:391-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogerson, S. J., R. Tembenu, C. Dobano, S. Plitt, T. E. Taylor, and M. E. Molyneux. 1999. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am. J. Trop. Med. Hyg. 61:467-472. [DOI] [PubMed] [Google Scholar]
- 58.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 59.Salanti, A., M. Dahlback, L. Turner, M. A. Nielsen, L. Barfod, P. Magistrado, A. T. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. Jensen, M. P. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179-191. [DOI] [PubMed] [Google Scholar]
- 61.Sargeant, T. J., M. Marti, E. Caler, J. M. Carlton, K. Simpson, T. P. Speed, and A. F. Cowman. 2006. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 7:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scherf, A., B. Pouvelle, P. A. Buffet, and J. Gysin. 2001. Molecular mechanisms of Plasmodium falciparum placental adhesion. Cell Microbiol. 3:125-131. [DOI] [PubMed] [Google Scholar]
- 63.Schneider, A. G., and O. Mercereau-Puijalon. 2005. A new Apicomplexa-specific protein kinase family: multiple members in Plasmodium falciparum, all with an export signature. BMC Genomics 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith, J. D., A. G. Craig, N. Kriek, D. Hudson-Taylor, S. Kyes, T. Fagan, R. Pinches, D. I. Baruch, C. I. Newbold, and L. H. Miller. 2000. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA 97:1766-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith, J. D., B. Gamain, D. I. Baruch, and S. Kyes. 2001. Decoding the language of var genes and Plasmodium falciparum sequestration. Trends Parasitol. 17:538-545. [DOI] [PubMed] [Google Scholar]
- 66.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 67.Tuikue Ndam, N. G., A. Salanti, J. Y. Le-Hesran, G. Cottrell, N. Fievet, L. Turner, S. Sow, J. M. Dangou, T. Theander, and P. Deloron. 2006. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of senegalese pregnant women. J. Infect. Dis. 193:713-720. [DOI] [PubMed] [Google Scholar]
- 68.Vincensini, L., S. Richert, T. Blisnick, A. Van Dorsselaer, E. Leize-Wagner, T. Rabilloud, and C. Braun Breton. 2005. Proteomic analysis identifies novel proteins of the Maurer's clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol. Cell. Proteomics 4:582-593. [DOI] [PubMed] [Google Scholar]
- 69.Westfall, P. H., and S. Young. 1993. Resampling-based multiple testing: exampes and methods for p-value adjustment. John Wiley and Sons, New York, NY.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.