Abstract
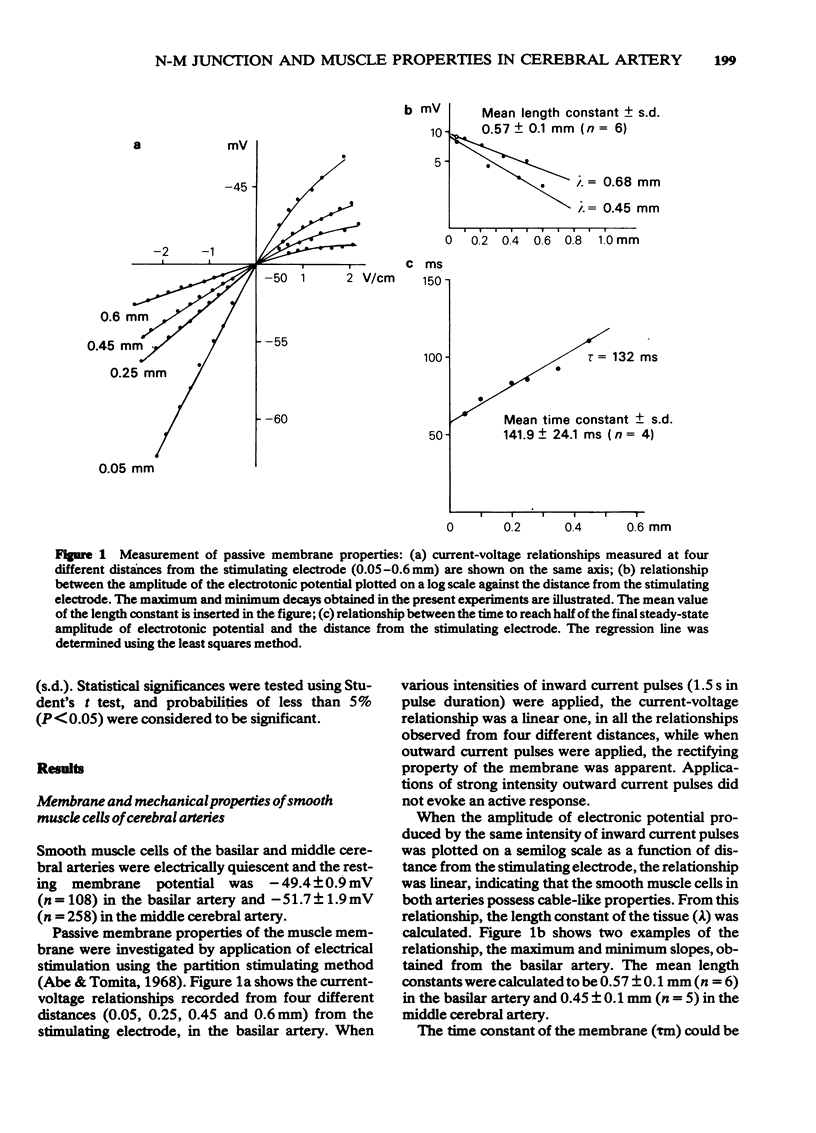
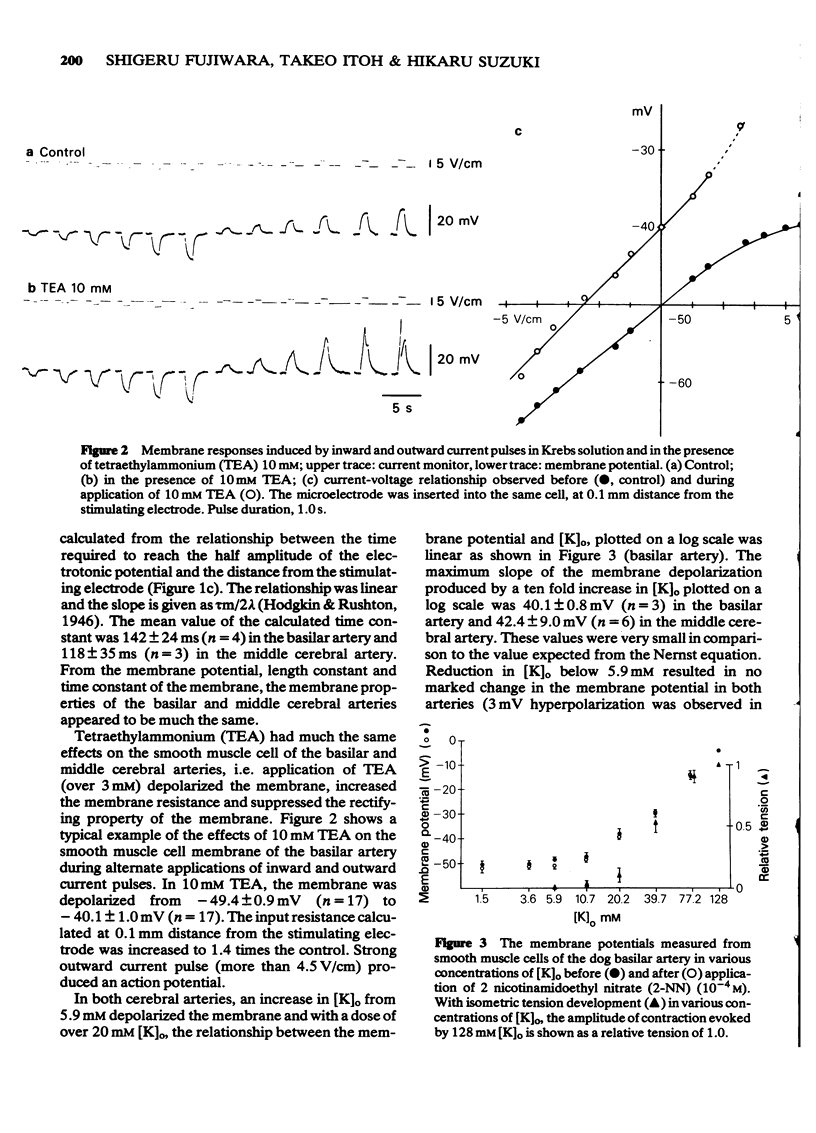
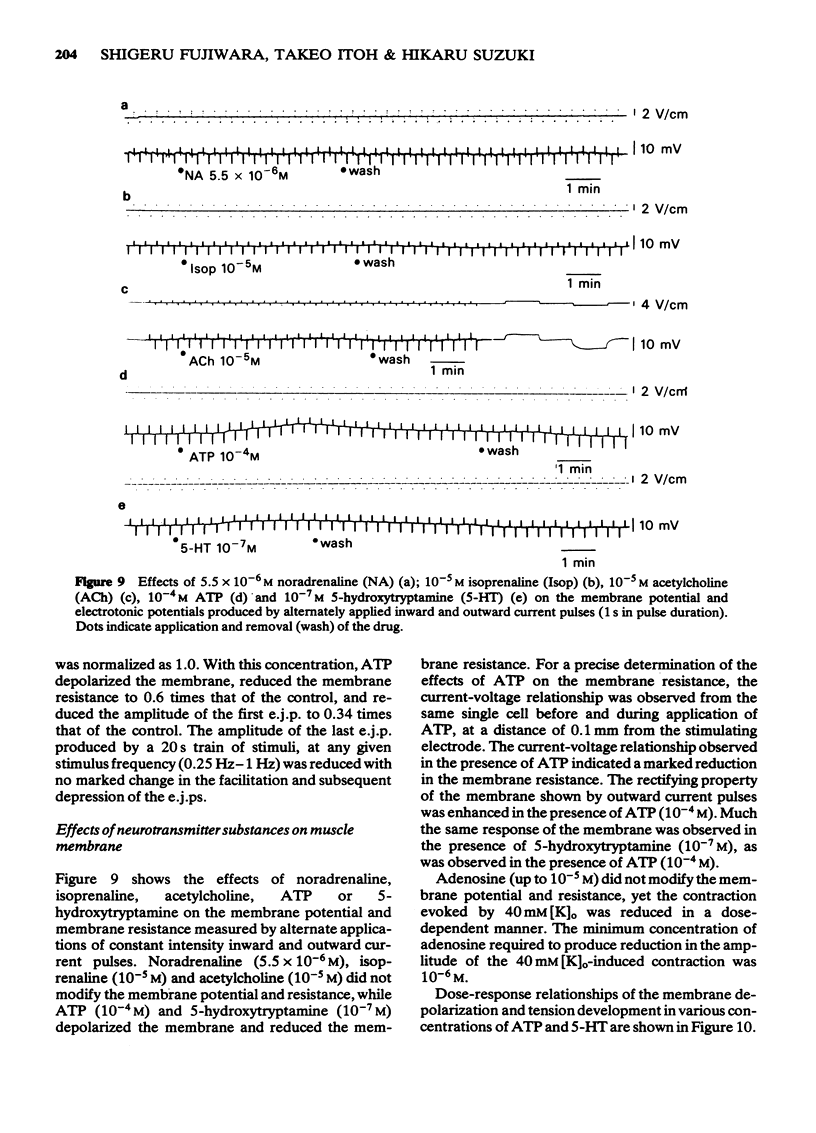
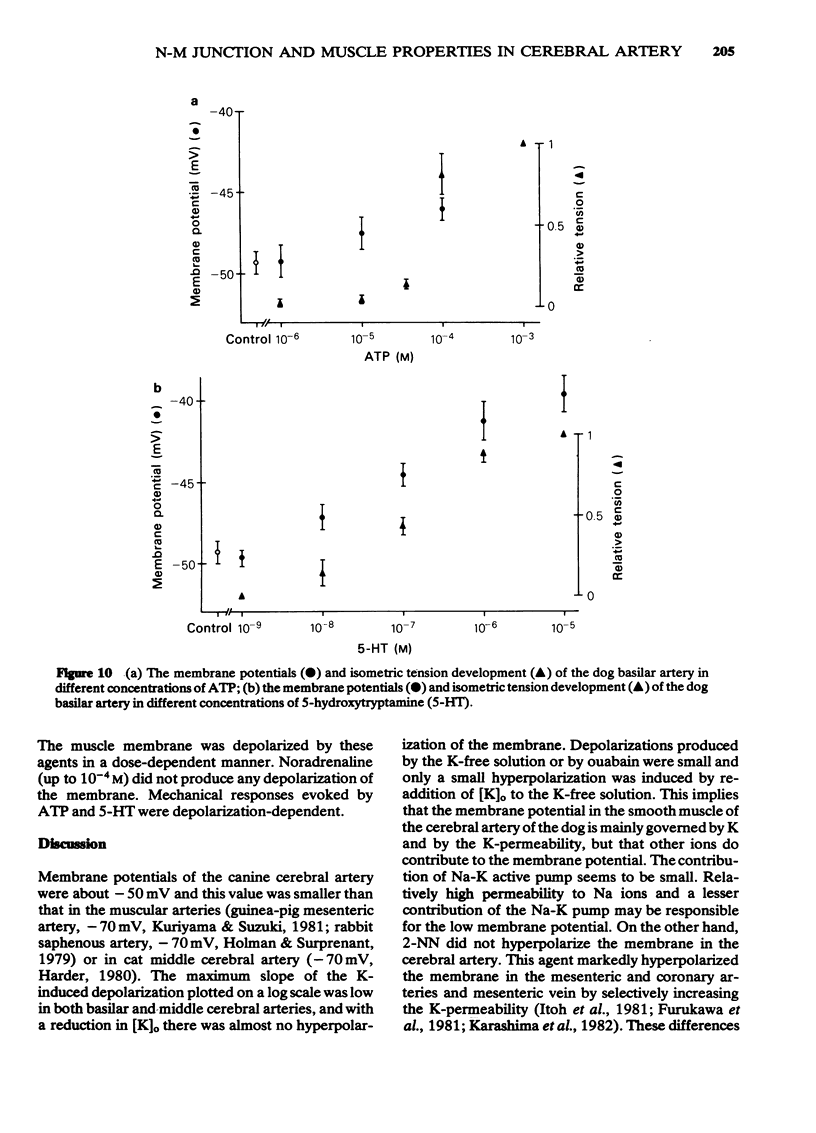
1 Drug actions on electrical and mechanical properties of smooth muscle cells and neuromuscular transmission in the canine cerebral arteries were investigated by use of microelectrode and isometric tension recording methods. 2 In the basilar and middle cerebral arteries, the resting membrane potentials were--49.4 mV and -51.7 mV, respectively, the length constants 0.57 mm and 0.45 mm, respectively and the time constants 142 ms and 118 ms, respectively. 3 Outward current pulses did not evoke the spike in either artery but did evoke the spike under conditions of pretreatment with 10 mM tetraethylammonium (TEA). 4 The maximum slope of depolarization produced by a ten fold increase in [K]o plotted on a log scale was 40.1 mV in the basilar artery and 42.2 mV in the middle cerebral artery. 2-Nicotinamidoethyl nitrate, the K-permeability accelerator, had no effect on the membrane potential. 5 K-free or ouabain [10(-5)M] treatment slightly depolarized the membrane. Re-addition of K [5.9 mM] hyperpolarized the membrane by several mV. Thus, the contribution of an active Na-K pump in the membrane potential seems to be small. 6 In both arteries, acetylcholine, adenosine, noradrenaline and isoprenaline in concentrations up to 10(-5)M did not modify the membrane potential and resistance, while 5-hydroxytryptamine (over 10(-8)M) and ATP (over 10(-5)M) depolarized the membrane, decreased the membrane resistance and produced a dose-dependent contraction. Adenosine suppressed the contraction evoked by excess [K]o (39.8 mM). 7 Perivascular nerve stimulation produced excitatory junction potentials (e.j.ps). Often e.j.ps were followed by a hyperpolarization. Repetitive stimulation produced facilitation after several stimuli and depression followed. In some cells, this depression appeared without facilitation. 8 The e.j.ps ceased with pretreatment with guanethidine (10(-6)M) or tetrodotoxin (3 X 10(-7)M), while phentolamine (10(-7)M) and yohimbine (10(-7)M) enhanced the amplitude of e.j.ps. ATP (10(-5)M) and noradrenaline (10(-6)M) suppressed and prazosin had little effect on the e.j.ps. Atropine (10(-6)M) also had no effect on the e.j.ps. 9 Specific features of the cerebral artery and systemic vascular beds were compared, and the features of adrenoceptors on the smooth muscle membrane were compared with findings in other vascular beds.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
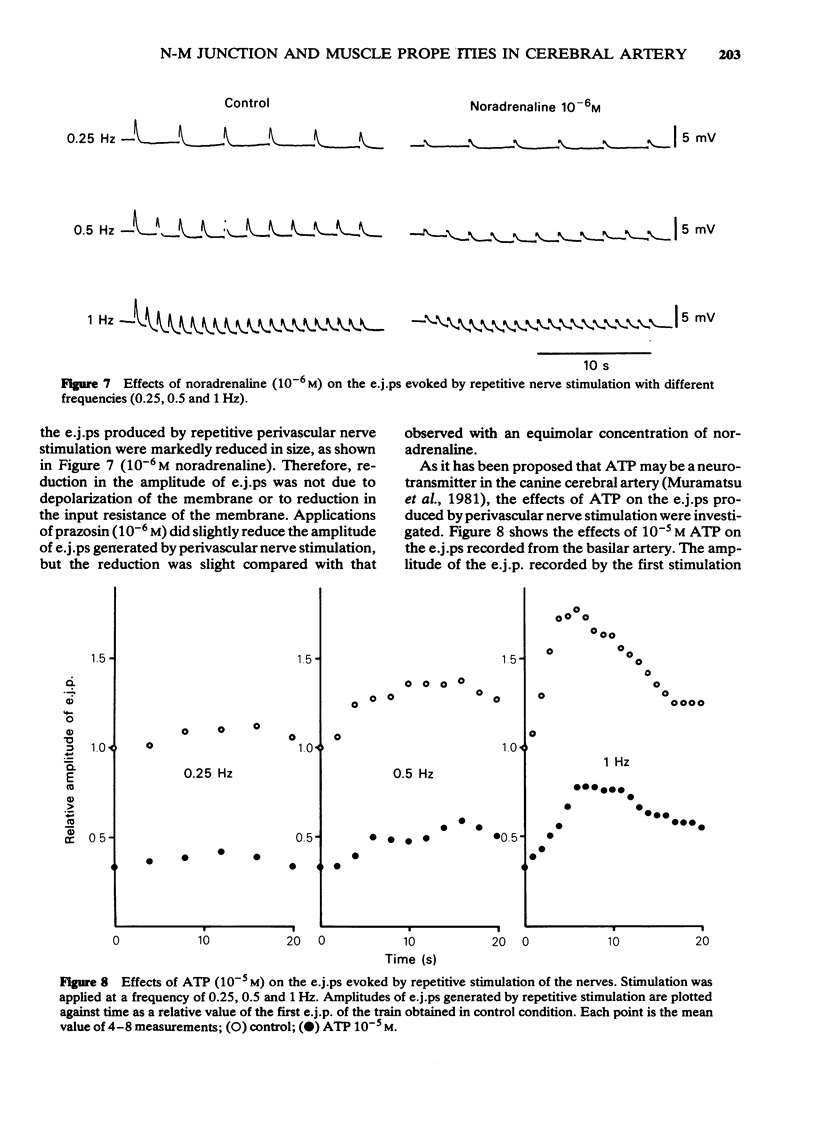
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
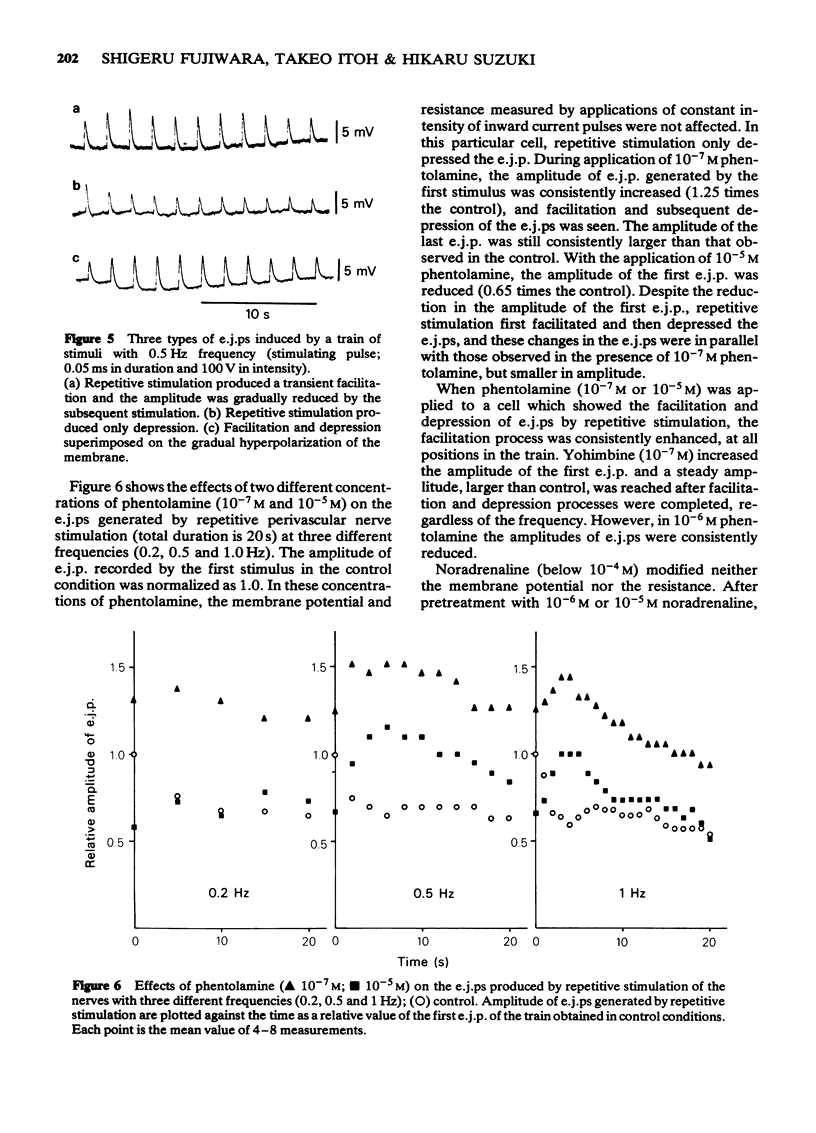
- Bevan J. A. A comparison of the contractile responses of the rabbit basilar and pulmonary arteries to sympathomimetic agonists: further evidence for variation in vascular adrenoceptor characteristics. J Pharmacol Exp Ther. 1981 Jan;216(1):83–89. [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalske H. F., Harakal C., Sevy R. W., Menkowitz B. J. Catecholamine content and response to norepinephrine of middle cerebral artery. Proc Soc Exp Biol Med. 1974 Jul;146(3):718–721. doi: 10.3181/00379727-146-38179. [DOI] [PubMed] [Google Scholar]
- Duckles S. P., Bevan J. A. Pharmacological characterization of adrenergic receptors of a rabbit cerebral artery in vitro. J Pharmacol Exp Ther. 1976 May;197(2):371–378. [PubMed] [Google Scholar]
- Duckles S. P., Bevan J. A. Responses of small rabbit pial arteries in vitro. Blood Vessels. 1979;16(2):80–86. [PubMed] [Google Scholar]
- Duckles S. P. Functional activity of the noradrenergic innervation of large cerebral arteries. Br J Pharmacol. 1980 Jun;69(2):193–199. doi: 10.1111/j.1476-5381.1980.tb07890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Owman C., Sjöberg N. O. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976 Oct 22;115(3):377–393. doi: 10.1016/0006-8993(76)90356-5. [DOI] [PubMed] [Google Scholar]
- Florence V. M., Bevan J. A. Biochemical determinations of cholinergic innervation in cerebral arteries. Circ Res. 1979 Aug;45(2):212–218. doi: 10.1161/01.res.45.2.212. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Ito Y., Itoh T., Kuriyama H., Suzuki H. Diltiazem-induced vasodilatation of smooth muscle cells of the canine basilar artery. Br J Pharmacol. 1982 Mar;75(3):455–467. doi: 10.1111/j.1476-5381.1982.tb09162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Itoh T., Kajiwara M., Kitamura K., Suzuki H., Ito Y., Kuriyama H. Vasodilating actions of 2-nicotinamidoethyl nitrate on porcine and guinea-pig coronary arteries. J Pharmacol Exp Ther. 1981 Jul;218(1):248–259. [PubMed] [Google Scholar]
- Harder D. R. Comparison of electrical properties of middle cerebral and mesenteric artery in cat. Am J Physiol. 1980 Jul;239(1):C23–C26. doi: 10.1152/ajpcell.1980.239.1.C23. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Furukawa K., Kajiwara M., Kitamura K., Suzuki H., Ito Y., Kuriyama H. Effects of 2-nicotinamidoethyl nitrate on smooth muscle cells and on adrenergic transmission in the guinea-pig and porcine mesenteric arteries. J Pharmacol Exp Ther. 1981 Jul;218(1):260–270. [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T., Itoh T., Kuriyama H. Effects of 2-nicotinamidoethyl nitrate on smooth muscle cells of the guinea-pig mesenteric and portal veins. J Pharmacol Exp Ther. 1982 May;221(2):472–480. [PubMed] [Google Scholar]
- Karashima T., Kuriyama H. Electrical properties of smooth muscle cell membrane and neuromuscular transmission in the guinea-pig basilar artery. Br J Pharmacol. 1981 Oct;74(2):495–504. doi: 10.1111/j.1476-5381.1981.tb09996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. J., Chiueh C. C., Adams M. Synaptic transmission of vasoconstrictor nerves in rabbit basilar artery. Eur J Pharmacol. 1980 Jan 11;61(1):55–70. doi: 10.1016/0014-2999(80)90381-7. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Hume W. R., Su C., Bevan J. A. Neurogenic vasodilation of cat cerebral arteries. Circ Res. 1978 Apr;42(4):535–542. doi: 10.1161/01.res.42.4.535. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Su C., Bevan J. A. Neurogenic sympathetic vasoconstriction of the rabbit basilar artery. Circ Res. 1976 Jul;39(1):120–126. doi: 10.1161/01.res.39.1.120. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Fujiwara M., Osumi Y., Shibata S. Vasoconstrictor and dilator actions of nicotine and electrical transmural stimulation on isolated dog cerebral arteries. Blood Vessels. 1978;15(1-3):110–118. doi: 10.1159/000158157. [DOI] [PubMed] [Google Scholar]
- Owman C., Edvinsson L., Nielsen K. C. Autonomic neuroreceptor mechanisms in brain vessels. Blood Vessels. 1974;11(1-2):2–31. doi: 10.1159/000157996. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effects of endogenous and exogenous noradrenaline on the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1981 Dec;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kuriyama H. Observation of quantal release of noradrenaline from vascular smooth muscles in potassium-free solution. Jpn J Physiol. 1980;30(4):665–670. doi: 10.2170/jjphysiol.30.665. [DOI] [PubMed] [Google Scholar]
- Toda N., Fujita Y. Responsiveness of isolated cerebral and peripheral arteries to serotonin, norepinephrine, and transmural electrical stimulation. Circ Res. 1973 Jul;33(1):98–104. doi: 10.1161/01.res.33.1.98. [DOI] [PubMed] [Google Scholar]
- Uchida E., Bohr D. F., Hoobler S. W. A method for studying isolated resistance vessels from rabbit mesentery and brain and their responses to drugs. Circ Res. 1967 Oct;21(4):525–536. doi: 10.1161/01.res.21.4.525. [DOI] [PubMed] [Google Scholar]


