Abstract
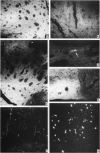
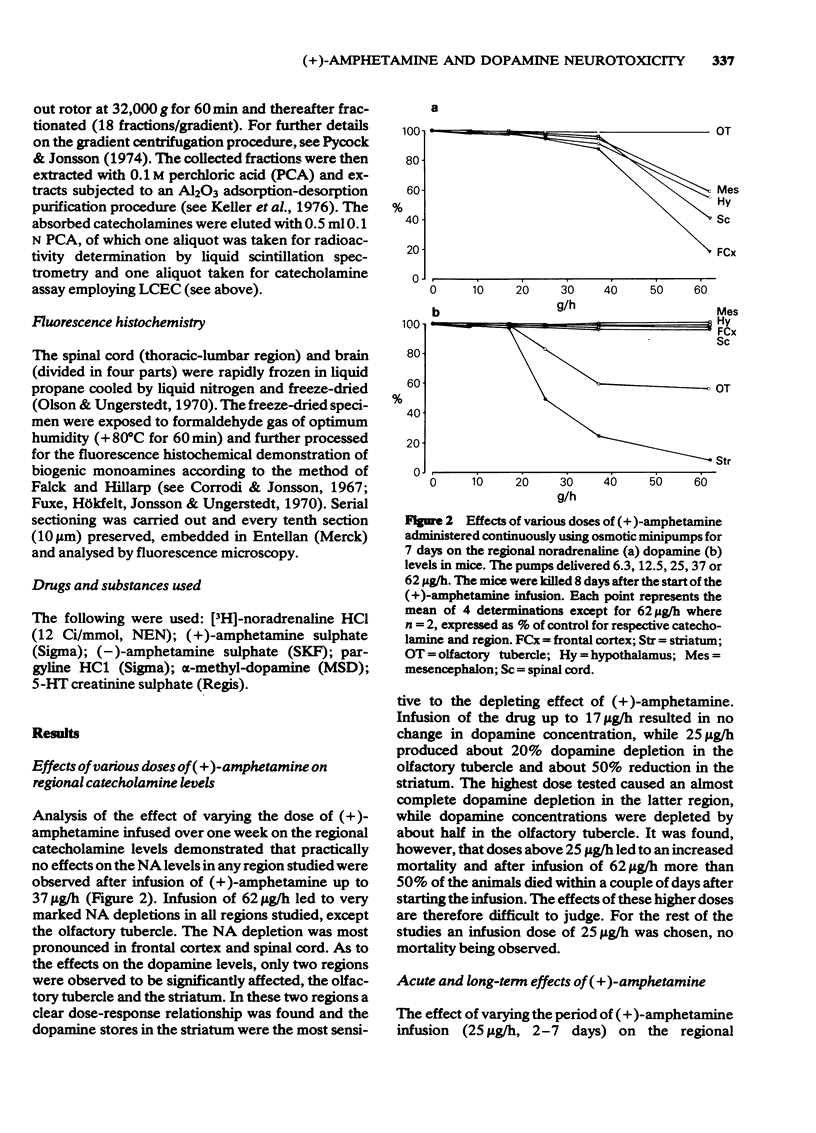
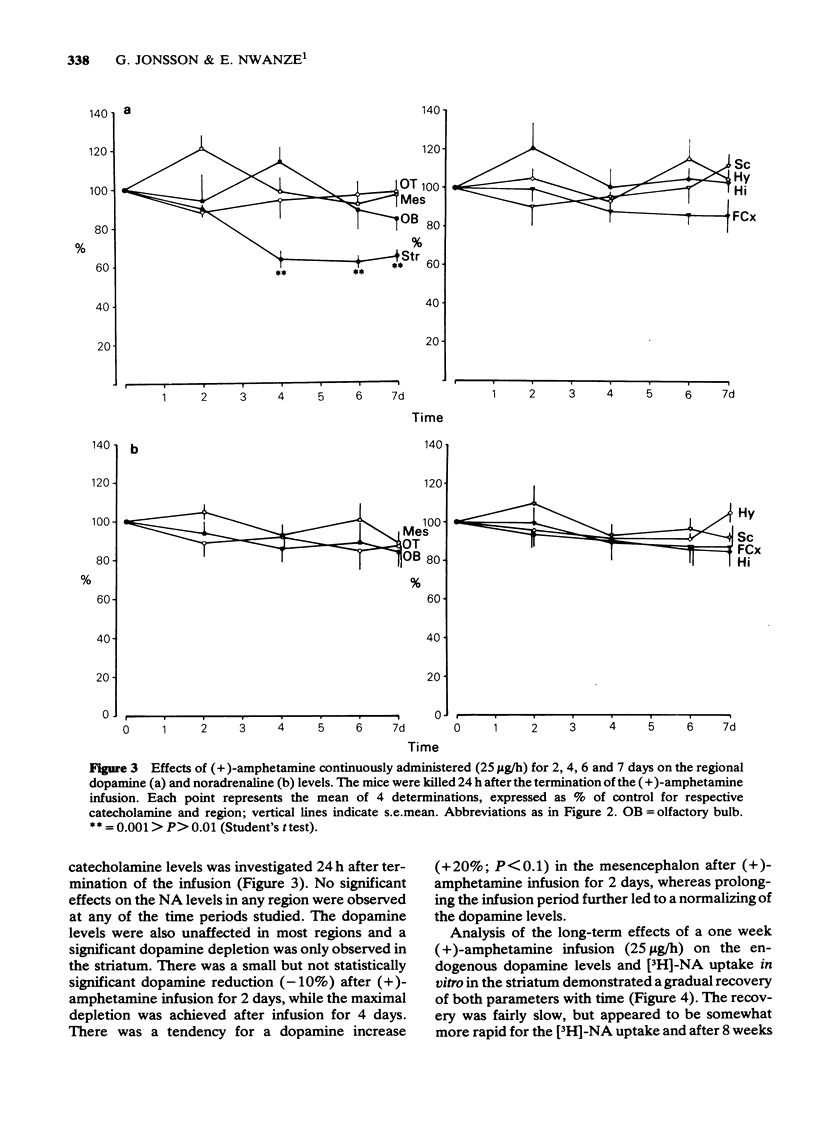
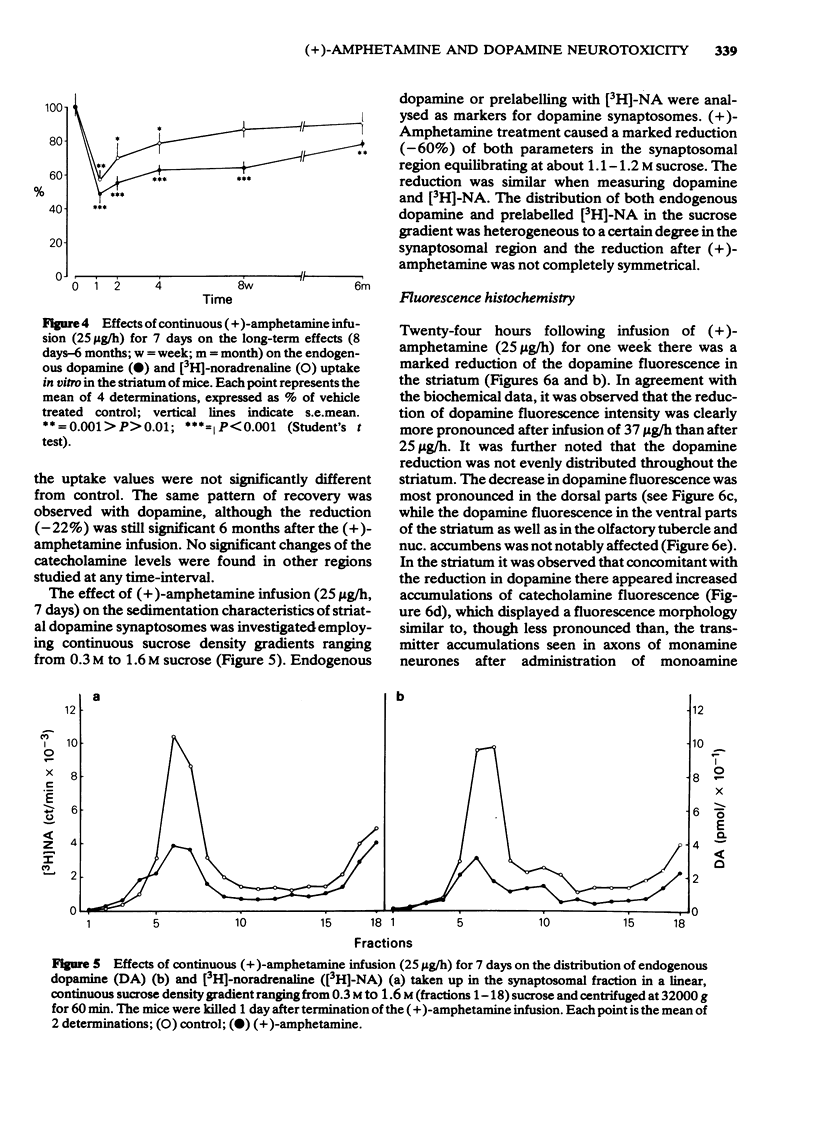
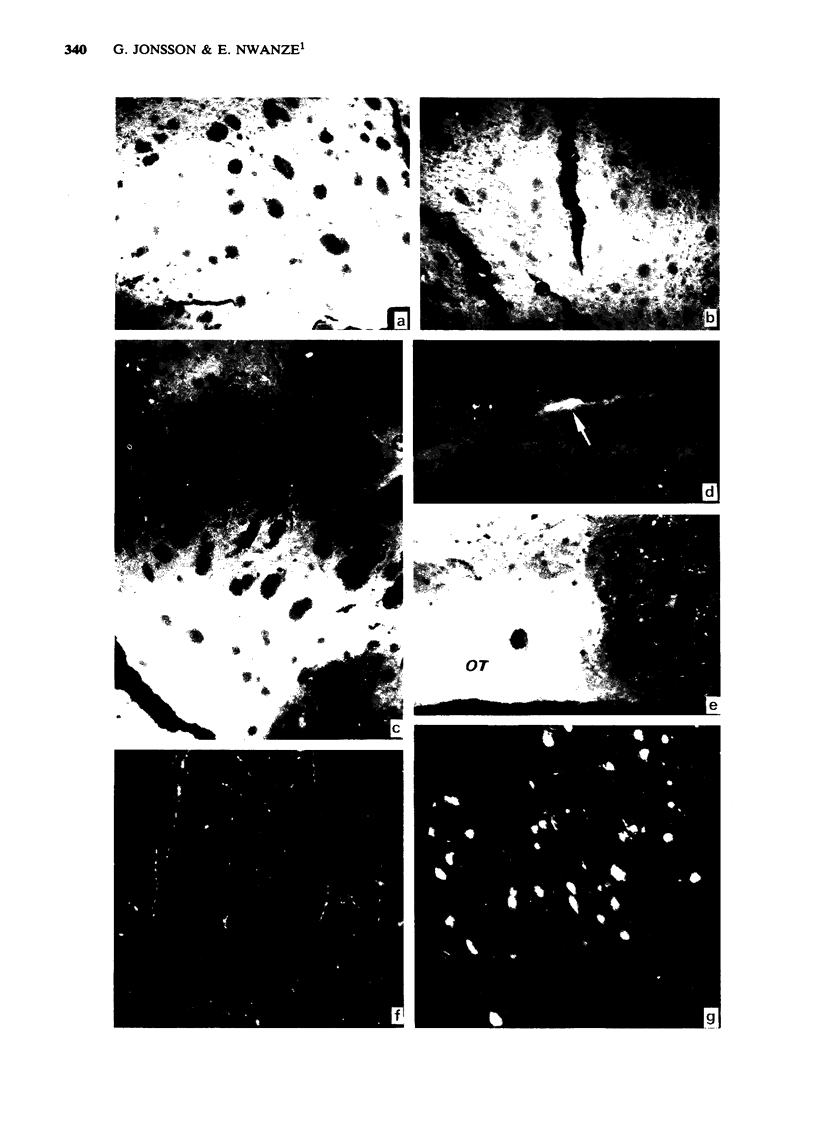
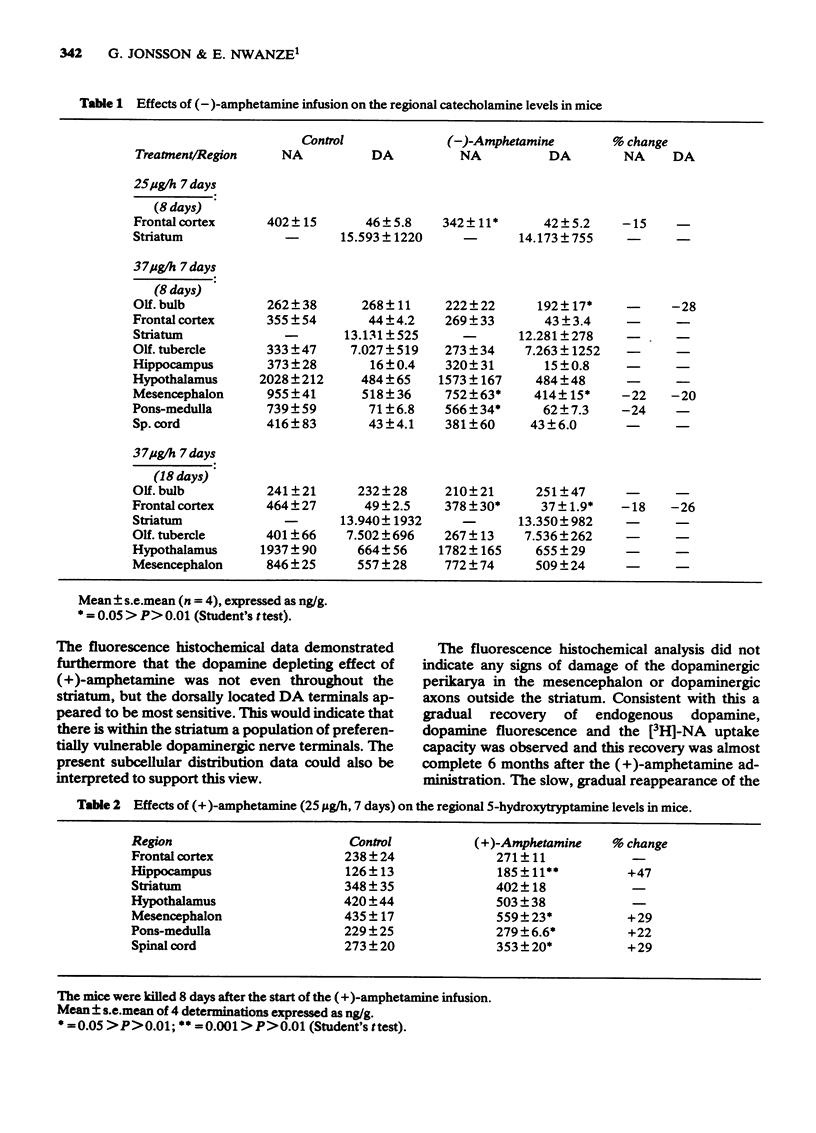
1 Infusion of large doses of (+)-amphetamine continuously for 7 days by means of osmotic minipumps caused a long-lasting reduction of endogenous dopamine levels, dopamine nerve terminals demonstrated histochemically and [3H]-noradrenaline uptake in vitro in the striatum of mice. 2 The effect was dose-dependent, fully developed after 4 days and selective for striatal dopamine up to a dose of (+)-amphetamine of 25 microgram/h. Higher doses, which produced increased mortality, also affected dopamine levels in the olfactory tubercle as well as noradrenaline in several regions. 3 Fluorescence histochemical studies using the Falck-Hillarp technique disclosed catecholamine accumulations in the striatum after (+)-amphetamine; a sign of neurotoxic damage. No effects on the dopamine cell bodies were noted. There were also no indications of neurotoxic damage to noradrenaline or 5-hydroxytryptamine neurones induced by (+)-amphetamine. 4 Large doses of (-)-amphetamine were without effect, demonstrating that the long-lasting impairment of transmitter uptake-storage mechanism in striatal dopamine nerve terminals is selective for (+)-amphetamine. 5 There was a slow gradual recovery of endogenous dopamine and [3H]-noradrenaline uptake in the striatum with time, which was almost complete 6 months after the (+)-amphetamine administration. 6 The results give further evidence for the view that (+)-amphetamine in large doses can have a selective neurotoxic action on a vulnerable population of a dopamine nerve terminals in the striatum. The results suggest in addition that there is a slow regrowth and regeneration with time of damaged dopamine nerve terminals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björklund A., Stenevi U. Regeneration of monoaminergic and cholinergic neurons in the mammalian central nervous system. Physiol Rev. 1979 Jan;59(1):62–100. doi: 10.1152/physrev.1979.59.1.62. [DOI] [PubMed] [Google Scholar]
- Bunney B. S., Walters J. R., Kuhar M. J., Roth R. H., Aghajanian G. K. D & L amphetamine stereoisomers: comparative potencies in affecting the firing of central dopaminergic and noradrenergic neurons. Psychopharmacol Commun. 1975;1(2):177–190. [PubMed] [Google Scholar]
- Domer F. R., Sankar R., Cole S., Wellmeyer D. Dose-dependent, amphetamine-induced changes in permeability of the blood-brain barrier of normotensive and spontaneously hypertensive rats. Exp Neurol. 1980 Dec;70(3):576–585. doi: 10.1016/0014-4886(80)90184-3. [DOI] [PubMed] [Google Scholar]
- Ellison G., Eison M. S., Huberman H. S., Daniel F. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science. 1978 Jul 21;201(4352):276–278. doi: 10.1126/science.26975. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Hemrick-Luecke S. Long-lasting depletion of striatal dopamine by a single injection of amphetamine in iprindole-treated rats. Science. 1980 Jul 11;209(4453):305–307. doi: 10.1126/science.7384808. [DOI] [PubMed] [Google Scholar]
- Hotchkiss A. J., Gibb J. W. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980 Aug;214(2):257–262. [PubMed] [Google Scholar]
- Hotchkiss A. J., Morgan M. E., Gibb J. W. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979 Oct 15;25(16):1373–1378. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Fuxe K., Hökfelt T. On the catecholamine innervation of the hypothalamus, with special reference to the median eminence. Brain Res. 1972 May 26;40(2):271–281. doi: 10.1016/0006-8993(72)90133-3. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Sachs C. Neurochemical properties of adrenergic nerves regenerated after 6-hydroxydopamine. J Neurochem. 1972 Nov;19(11):2577–2585. doi: 10.1111/j.1471-4159.1972.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Keller R., Oke A., Mefford I., Adams R. N. Liquid chromatographic analysis of catecholamines routine assay for regional brain mapping. Life Sci. 1976 Oct 1;19(7):995–1003. doi: 10.1016/0024-3205(76)90290-3. [DOI] [PubMed] [Google Scholar]
- Kuczenski R., Segal D. S. Differential effects of D- and L-amphetamine and methylphenidate on rat striatal dopamine biosynthesis. Eur J Pharmacol. 1975 Feb;30(2):244–251. doi: 10.1016/0014-2999(75)90106-5. [DOI] [PubMed] [Google Scholar]
- Kuhn C. M., Schanberg S. M. Metabolism of amphetamine after acute and chronic administration to the rat. J Pharmacol Exp Ther. 1978 Nov;207(2):544–554. [PubMed] [Google Scholar]
- Lidbrink P., Jonsson G. Noradrenaline nerve terminals in the cerebral cortex: effects on noradrenaline uptake and storage following axonal lesion with 6-hydroxydopamine. J Neurochem. 1974 May;22(5):617–626. doi: 10.1111/j.1471-4159.1974.tb04272.x. [DOI] [PubMed] [Google Scholar]
- Lorez H. Fluorescence histochemistry indicates damage of striatal dopamine nerve terminals in rats after multiple doses of methamphetamine. Life Sci. 1981 Feb 23;28(8):911–916. doi: 10.1016/0024-3205(81)90053-9. [DOI] [PubMed] [Google Scholar]
- Nwanze E., Jonsson G. Amphetamine neurotoxicity on dopamine nerve terminals in the caudate nucleus of mice. Neurosci Lett. 1981 Oct 23;26(2):163–168. doi: 10.1016/0304-3940(81)90343-8. [DOI] [PubMed] [Google Scholar]
- Olson L., Ungerstedt U. A simple high capacity freeze-drier for histochemical use. Histochemie. 1970;22(1):8–19. doi: 10.1007/BF00310544. [DOI] [PubMed] [Google Scholar]
- Ponzio F., Jonsson G. A rapid and simple method for the determination of picogram levels of serotonin in brain tissue using liquid chromatography with electrochemical detection. J Neurochem. 1979 Jan;32(1):129–132. doi: 10.1111/j.1471-4159.1979.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Pycock C., Jonsson G. Transmitter-dependent changes of equilibrium characteristics of catecholamine synaptosomes in sucrose density gradients. Med Biol. 1974 Aug;52(4):260–268. [PubMed] [Google Scholar]
- Ricaurte G. A., Schuster C. R., Seiden L. S. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980 Jul 7;193(1):153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Sanders-Bush E. Long-term effects of continuous exposure to amphetamine on brain dopamine concentration and synaptosomal uptake in mice. Eur J Pharmacol. 1980 Aug 8;65(4):439–443. doi: 10.1016/0014-2999(80)90351-9. [DOI] [PubMed] [Google Scholar]
- Steranka L. R. Stereospecific long-term effects of amphetamine on striatal dopamine neurons in rats. Eur J Pharmacol. 1981 Dec 17;76(4):443–446. doi: 10.1016/0014-2999(81)90119-9. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Jacobs B. L. Chronic amphetamine administration to cats: behavioral and neurochemical evidence for decreased central serotonergic function. J Pharmacol Exp Ther. 1979 Nov;211(2):375–384. [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968 Dec;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Wagner G. C., Ricaurte G. A., Seiden L. S., Schuster C. R., Miller R. J., Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980 Jan 6;181(1):151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]