Abstract
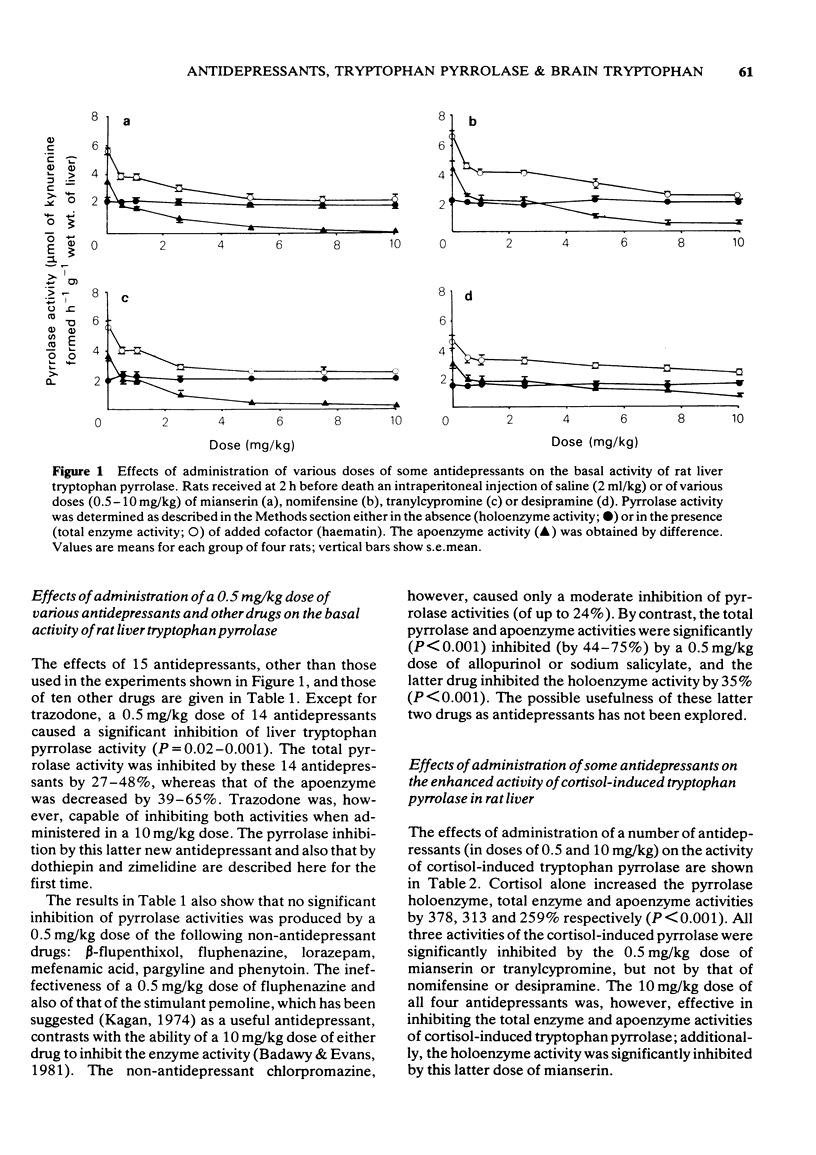
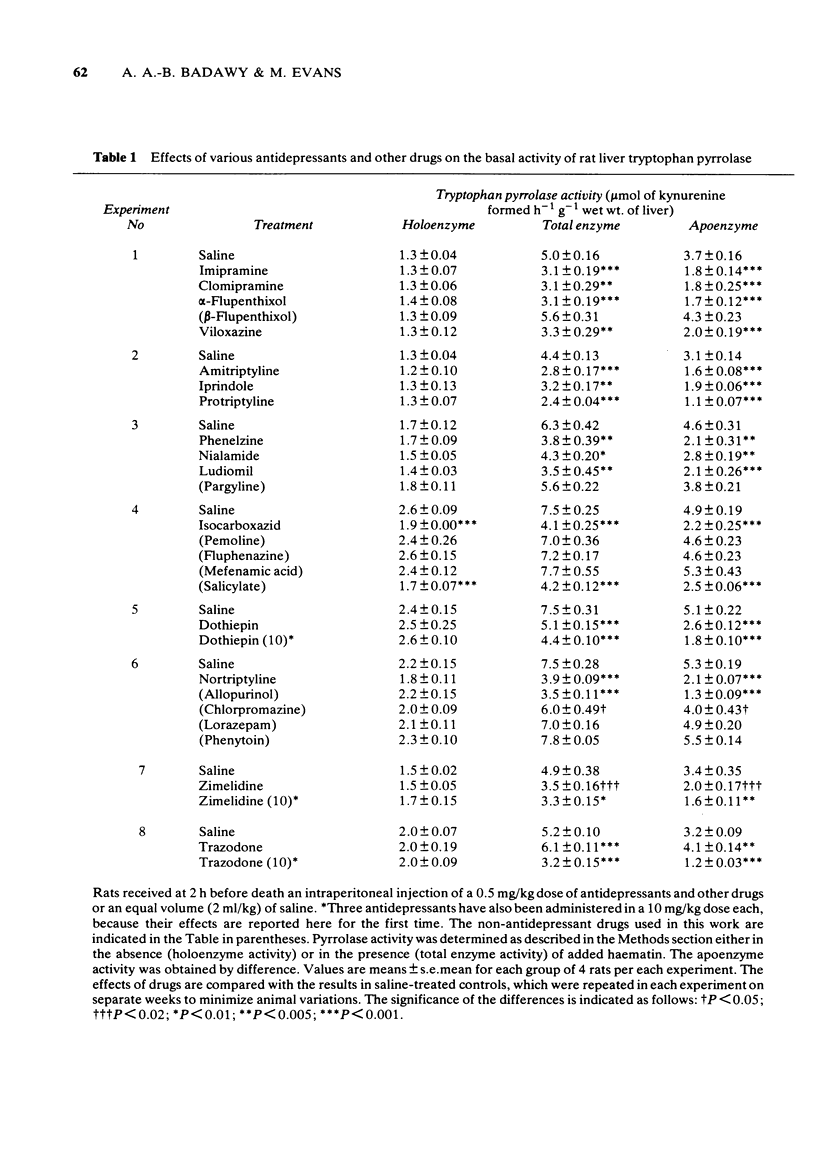
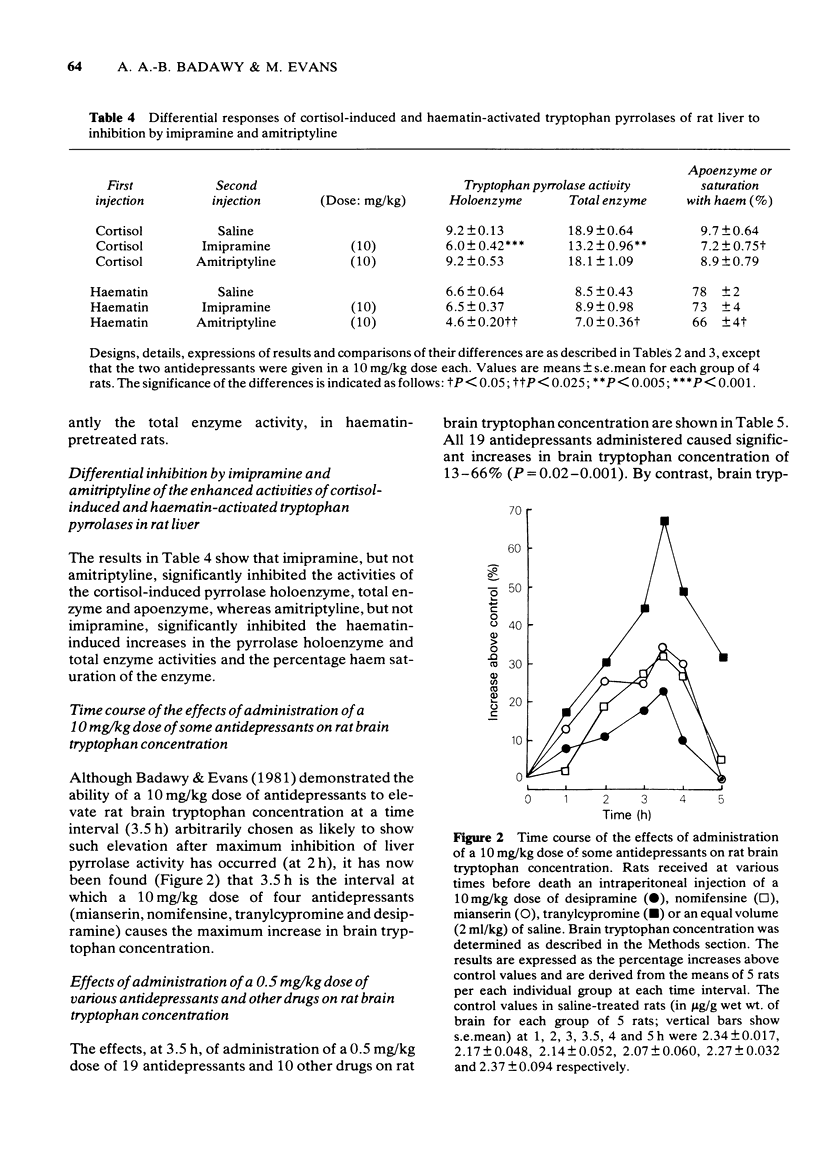
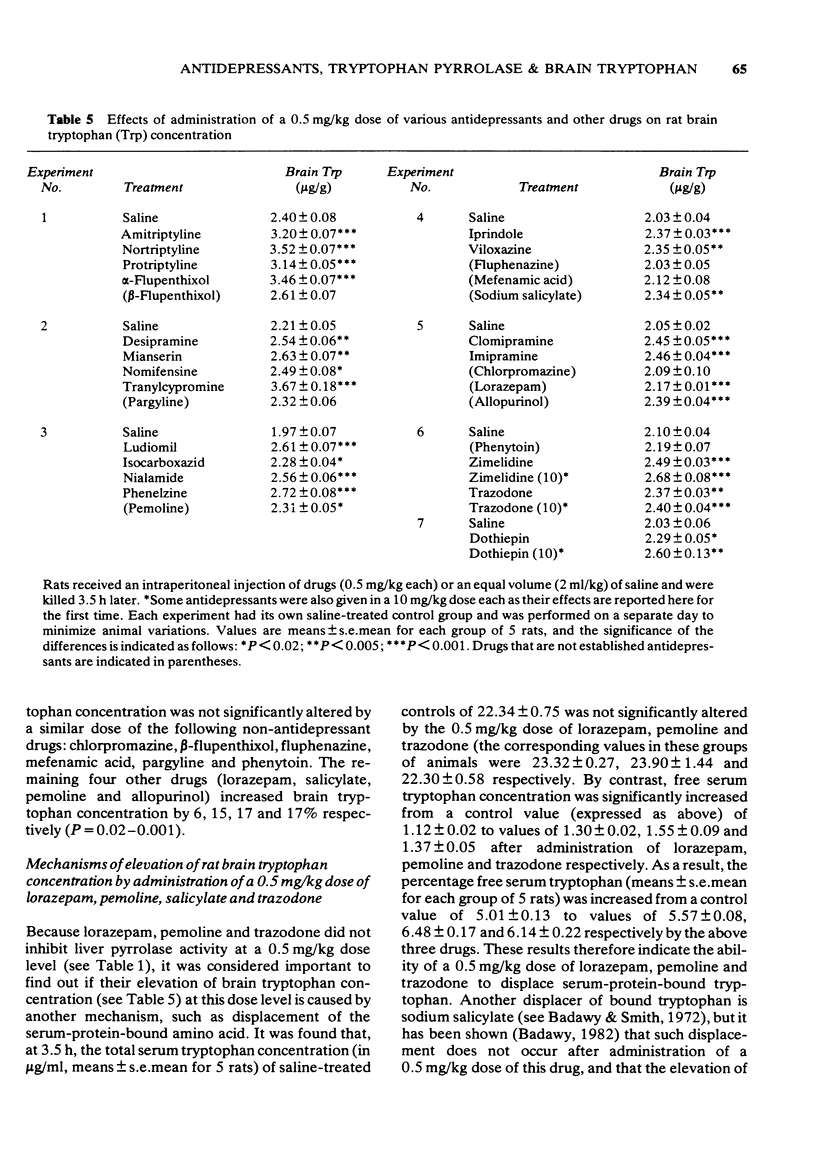
1 Administration to rats of a 0.5 mg/kg dose of any of 19 antidepressants, but not that of many other drugs, causes a significant inhibition of the total enzyme and apoenzyme activities of liver tryptophan pyrrolase (of 24-48% and 37-65% respectively) and elevates brain tryptophan concentration by 13-66%. 2 When liver tryptophan pyrrolase activity is enhanced by pretreatment with cortisol or haematin, subsequent administration of a 0.5 mg/kg dose of some, but not other, antidepressants causes inhibition, which is weak (up to 38%). 3 This weak inhibition of the enhanced pyrrolase activity together with other pharmacological and physiological factors could explain the time lag between the start of antidepressant medication and the occurrence of a therapeutic response. 4 The cortisol-induced and haematin-activated pyrrolases respond differentially to inhibition by imipramine and amitriptyline, and this may explain the differential response to these two drugs of depressed patients in relation to urinary excretion of the noradrenaline metabolite 3-methoxy-4-hydroxyphenylglycol. 5 The results are discussed in relation to the mechanism of action of antidepressants and the possible involvement of disturbed hepatic tryptophan metabolism in depressive illness.
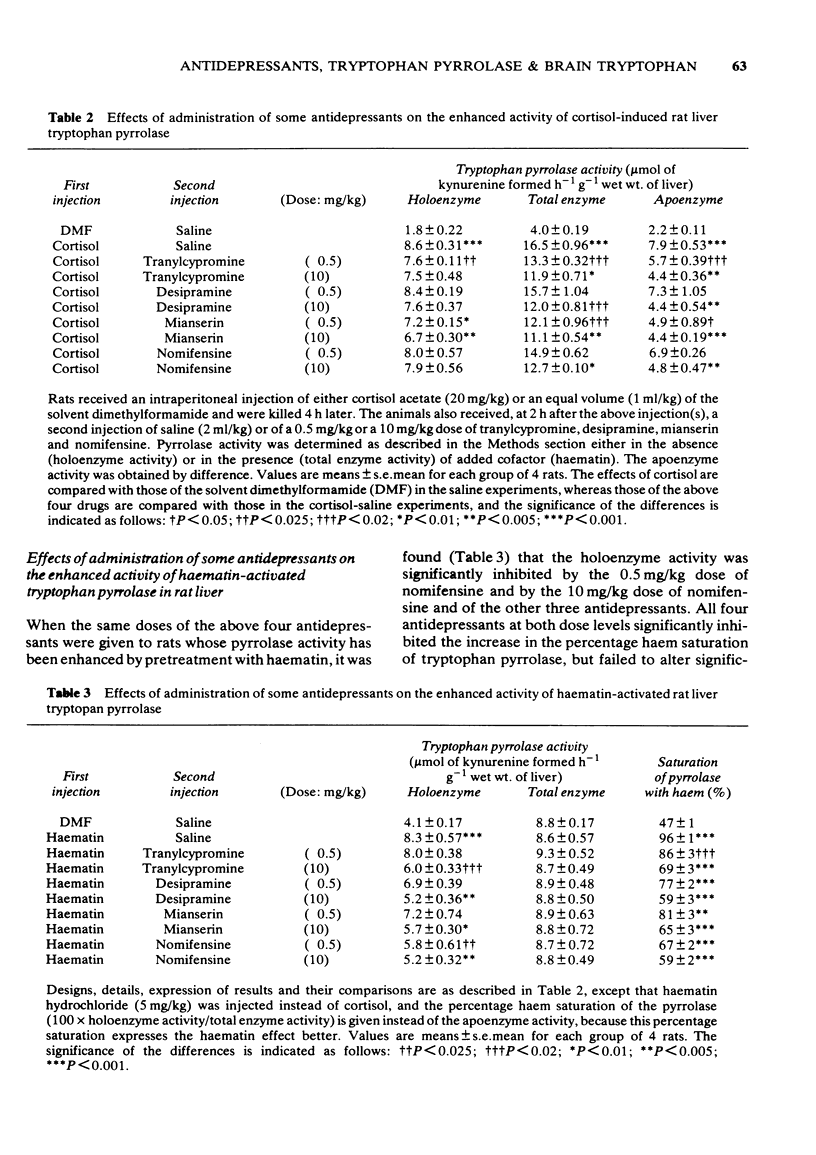
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badawy A. A., Evans M. Animal liver tryptophan pyrrolases: Absence of apoenzyme and of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem J. 1976 Jul 15;158(1):79–88. doi: 10.1042/bj1580079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. Inhibition of rat liver tryptophan pyrrolase activity and elevation of brain tryptophan concentration by administration of antidepressants. Biochem Pharmacol. 1981 Jun 1;30(11):1211–1216. doi: 10.1016/0006-2952(81)90299-9. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: Experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975 Sep;150(3):511–520. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. Mechanisms of elevation of rat brain tryptophan concentration by various doses of salicylate. Br J Pharmacol. 1982 May;76(1):211–213. doi: 10.1111/j.1476-5381.1982.tb09208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Smith M. J. Changes in liver tryptophan and tryptophan pyrrolase activity after administration of salicylate and tryptophan to the rat. Biochem Pharmacol. 1972 Jan;21(1):97–101. doi: 10.1016/0006-2952(72)90254-7. [DOI] [PubMed] [Google Scholar]
- Bloxam D. L., Warren W. H. Error in the determination of tryptophan by the method of Denkla and Dewey. A revised procedure. Anal Biochem. 1974 Aug;60(2):621–625. doi: 10.1016/0003-2697(74)90275-9. [DOI] [PubMed] [Google Scholar]
- Carroll B. J., Curtis G. C., Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry. 1976 Sep;33(9):1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- Cazzullo C. L., Mangoni A., Mascherpa G. Tryptophan metabolism in affective psychoses. Br J Psychiatry. 1966 Feb;112(483):157–162. doi: 10.1192/bjp.112.483.157. [DOI] [PubMed] [Google Scholar]
- Curzon G., Green A. R. Rapid method for the determination of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in small regions of rat brain. Br J Pharmacol. 1970 Jul;39(3):653–655. doi: 10.1111/j.1476-5381.1970.tb10373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G. Relationships between stress and brain 5-hydroxytryptamine and their possible significance in affective disorders. J Psychiatr Res. 1972 Sep;9(3):243–252. doi: 10.1016/0022-3956(72)90023-4. [DOI] [PubMed] [Google Scholar]
- Denckla W. D., Dewey H. K. The determination of tryptophan in plasma, liver, and urine. J Lab Clin Med. 1967 Jan;69(1):160–169. [PubMed] [Google Scholar]
- Kagan G. Clinical trial of pemoline in general practice. Br J Clin Pract. 1974 Nov;28(11):375–378. [PubMed] [Google Scholar]
- Mangoni A. The "kynurenine shunt" and depression. Adv Biochem Psychopharmacol. 1974;11(0):293–298. [PubMed] [Google Scholar]
- Rubin R. T. Adrenal cortical activity changes in manic-depressive illness. Influence on intermediary metabolism of tryptophan. Arch Gen Psychiatry. 1967 Dec;17(6):671–679. doi: 10.1001/archpsyc.1967.01730300031006. [DOI] [PubMed] [Google Scholar]
- Samsonova M. L., Lapin I. P. Antidepressants and liver tryptophan pyrrolase activity. Biochem Pharmacol. 1973 Jun 15;22(12):1499–1507. doi: 10.1016/0006-2952(73)90327-4. [DOI] [PubMed] [Google Scholar]
- Schildkraut J. J. Norepinephrine metabolites as biochemical criteria for classifying depressive disorders and predicting responses to treatment: preliminary findings. Am J Psychiatry. 1973 Jun;130(6):695–699. doi: 10.1176/ajp.130.6.695. [DOI] [PubMed] [Google Scholar]
- Schlesser M. A., Winokur G., Sherman B. M. Hypothalamic-pituitary-adrenal axis activity in depressive illness. Its relationship to classification. Arch Gen Psychiatry. 1980 Jul;37(7):737–743. doi: 10.1001/archpsyc.1980.01780200015001. [DOI] [PubMed] [Google Scholar]
- Young S. N. Mechanism of decline in rat brain 5-hydroxytryptamine after induction of liver tryptophan pyrrolase by hydrocortisone: roles of tryptophan catabolism and kynurenine synthesis. Br J Pharmacol. 1981 Nov;74(3):695–700. doi: 10.1111/j.1476-5381.1981.tb10480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


