Abstract
1 The effects of the putative 5-hydroxytryptamine (5-HT) receptor antagonists, methysergide, mianserin and methergoline, the dopamine receptor antagonists, haloperidol, thioridazine and clozapine, and the noradrenaline (NA) receptor antagonists, phentolamine, phenoxybenzamine and propranolol on the behavioural responses of mice to β-phenylethylamine (PEA, 75 mg/kg) have been examined.
2 PEA produced a syndrome consisting of three distinct phases. The brief initial phase (0-5 min after injection) which consisted of forward walking, sniffing and headweaving, was succeeded by a locomotor depressant phase (5-20 min after injection) which consisted of abortive grooming, headweaving, splayed hindlimbs, forepaw padding, sniffing and hyperreactivity, and a late locomotor stimulant phase (20-35 min after injection), which was characterized by forward walking, sniffing, hyperreactivity, rearing and licking.
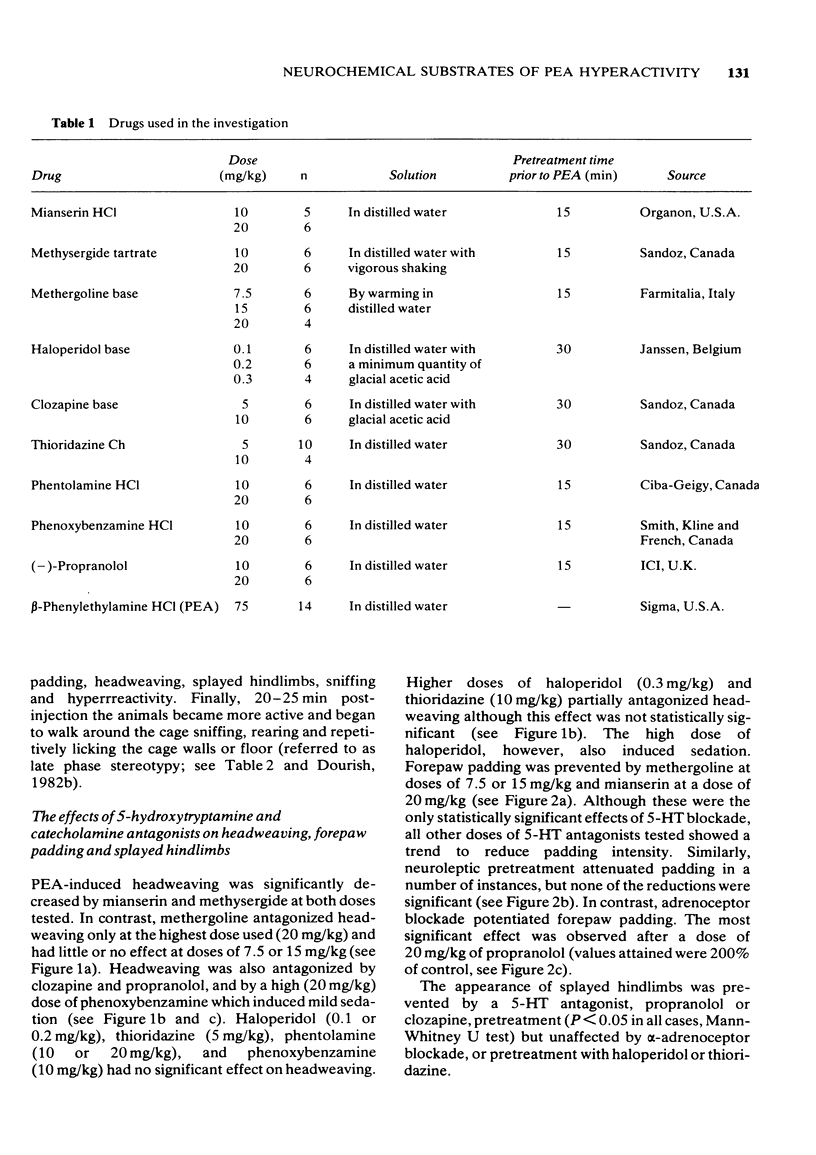
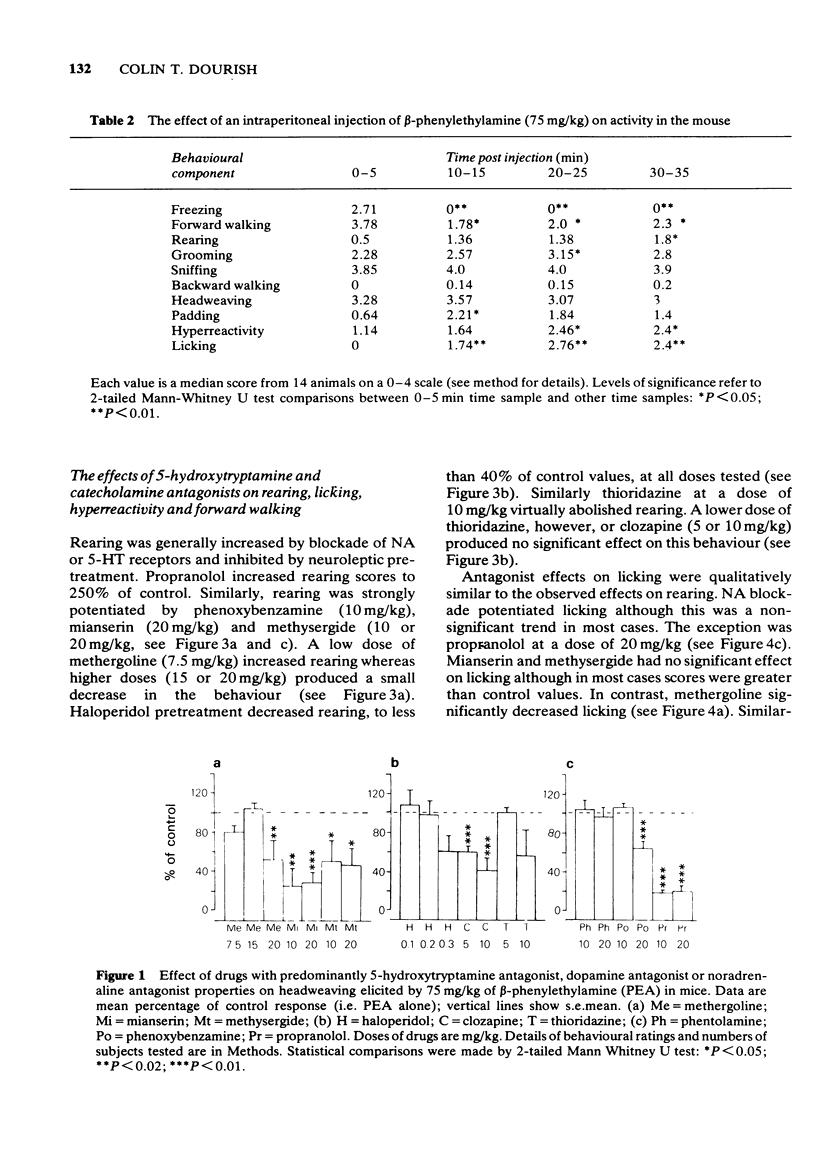
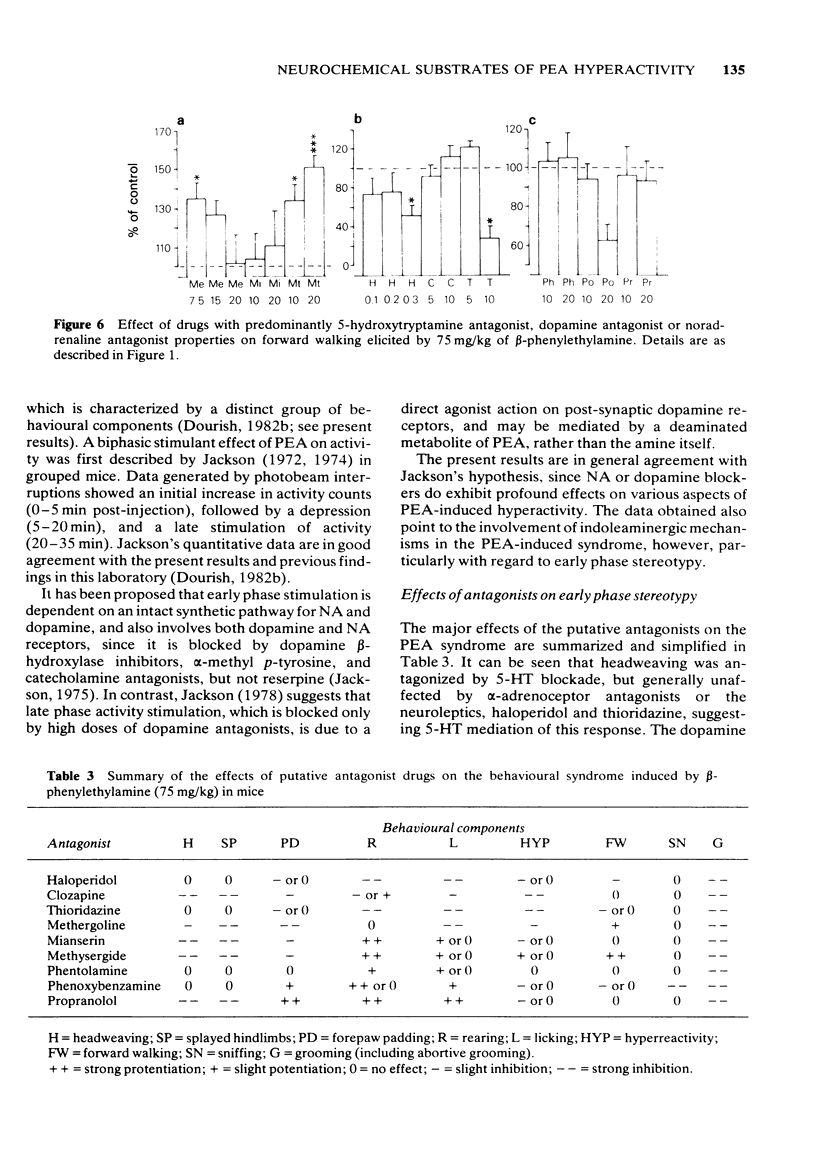
3 Methysergide, mianserin, methergoline, clozapine and propranolol inhibited headweaving and splayed hindlimbs, whereas haloperidol, thioridazine, phentolamine and phenoxybenzamine had no effect on these responses. Forepaw padding was strongly inhibited by methergoline and a high dose of mianserin, and weakly antagonized by methysergide, clozapine, haloperidol and thioridazine. In contrast, padding was mildly potentiated by phenoxybenzamine and phentolamine but strongly potentiated by propranolol. It is proposed that headweaving and splayed hindlimbs are 5-HT-mediated responses whereas forepaw padding also involves 5-HT mechanisms but may be partially due to release of tryptamine.
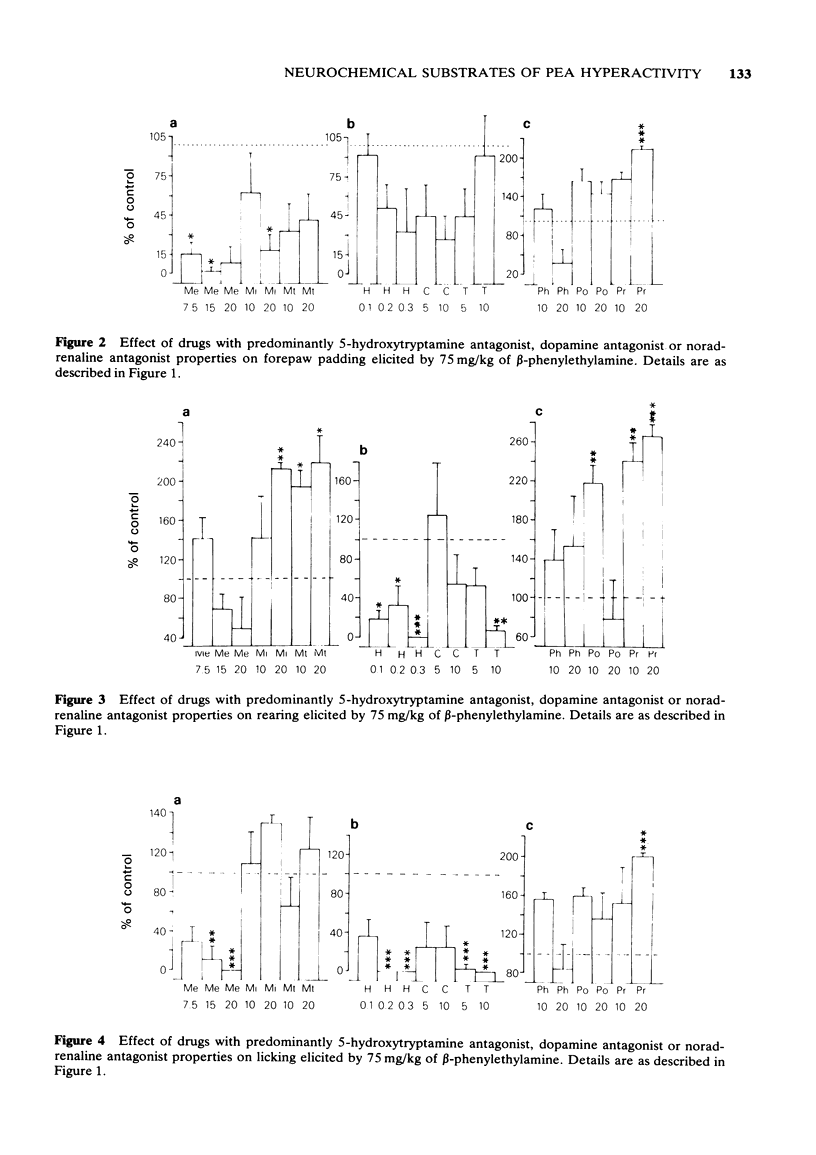
4 Rearing and licking were inhibited by haloperidol (most strongly), thioridazine and clozapine but potentiated by mianserin, methysergide, propranolol, phenoxybenzamine or phentolamine. Methergoline inhibited licking without affecting rearing. It is suggested that PEA-induced rearing and licking are produced by activation of dopaminergic neurones and inhibited by 5-HT or NA stimulation.
5 Phenoxybenzamine inhibited sniffing and produced backward walking when administered prior to PEA, suggesting mediation by NA of sniffing and an inhibitory influence of NA on backward walking.
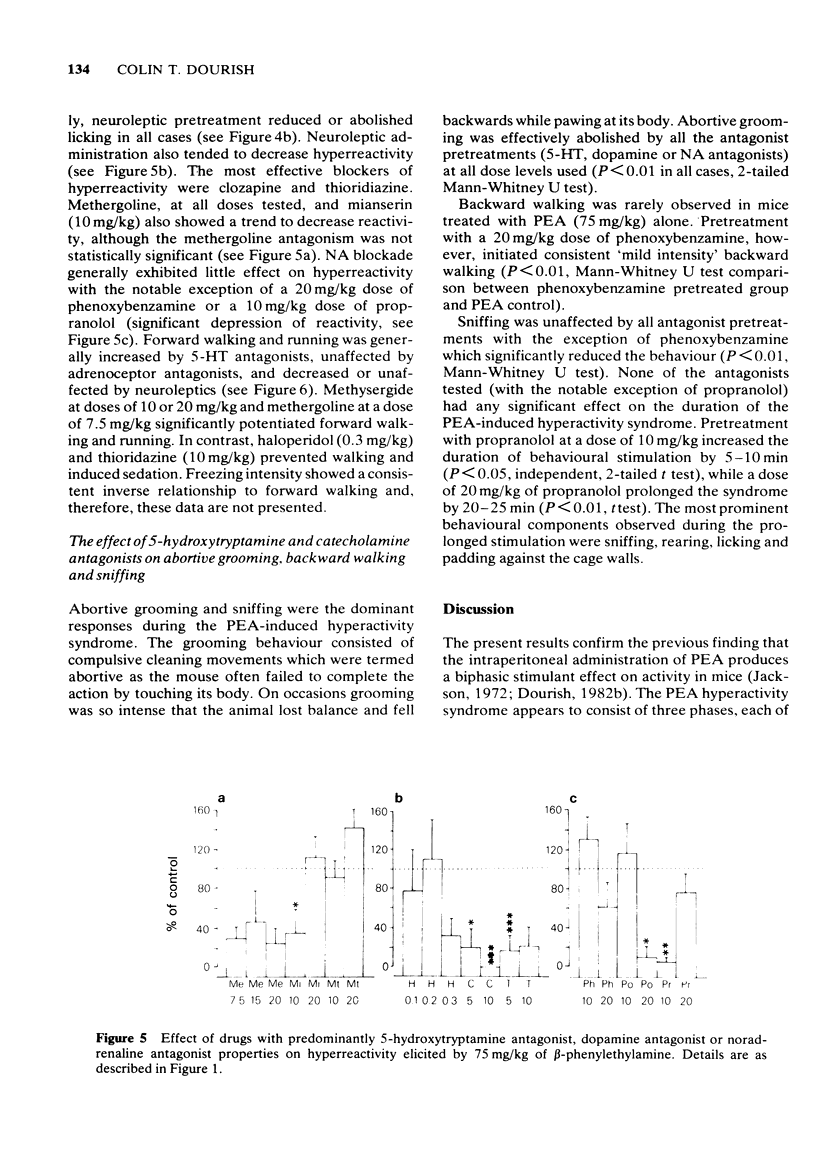
6 Clozapine and thioridazine were the most effective antagonists of hyperreactivity and it is proposed that this response is dopamine-mediated. Forward walking was inhibited by high doses of haloperidol or clozapine and potentiated by methergoline, mianserin or methysergide, suggesting that hyperactivity may also be mediated by dopamine but subject to 5-HT inhibition.
7 Abortive grooming was the dominant behavioural component observed after PEA administration and was prevented by all of the antagonists tested which suggests that catecholamine and 5-HT mechanisms may be involved in the expression of this response.
8 Since PEA is an endogenous compound in animals and man, and has been claimed to be present in abnormal amounts in some schizophrenics, PEA-induced behavioural stimulation in mice (which includes the postulated hallucinogenic responses of abortive grooming and backward walking) may be a useful animal model of psychosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borison R. L., Diamond B. I. A new animal model for schizophrenia: interactions with adrenergic mechanisms. Biol Psychiatry. 1978 Apr;13(2):217–225. [PubMed] [Google Scholar]
- Borison R. L., Havdala H. S., Diamond B. I. Chronic phenylethylamine stereotypy in rats: a new animal model for schizophrenia? Life Sci. 1977 Jul 1;21(1):117–122. doi: 10.1016/0024-3205(77)90431-3. [DOI] [PubMed] [Google Scholar]
- Boulton A. A., Juorio A. V., Philips S. R., Wu P. H. Some arylalkylamines in rabbit brain. Brain Res. 1975 Oct 10;96(1):212–216. doi: 10.1016/0006-8993(75)90600-9. [DOI] [PubMed] [Google Scholar]
- Boulton A. A., Milward L. Separation, detection and quantitative analysis of urinary beta-phenylethylamine. J Chromatogr. 1971 May 6;57(2):287–296. doi: 10.1016/0021-9673(71)80042-0. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Andersen H., Randrup A. The monoamine oxidase B inhibitor deprenyl potentiates phenylethylamine behaviour in rats without inhibition of catecholamine metabolite formation. Eur J Pharmacol. 1975 Nov;34(1):181–187. doi: 10.1016/0014-2999(75)90238-1. [DOI] [PubMed] [Google Scholar]
- Braestrup C. Changes in drug-induced stereotyped behavior after 6-OHDA lesions in noradrenaline neurons. Psychopharmacology (Berl) 1977 Jan 31;51(2):199–204. doi: 10.1007/BF00431741. [DOI] [PubMed] [Google Scholar]
- Clineschmidt B. V., Lotti V. J. Indoleamine antagonists: relative potencies as inhibitors of tryptamine- and 5-hydroxytryptophan-evoked responses. Br J Pharmacol. 1974 Feb;50(2):311–313. doi: 10.1111/j.1476-5381.1974.tb08577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B., Lee T. F., Martin D. Different hypothalamic receptors mediate 5-hydroxytryptamine- and tryptamine-induced core temperature changes in the rat. Br J Pharmacol. 1981 Mar;72(3):477–482. doi: 10.1111/j.1476-5381.1981.tb10999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Deakin J. F. Role of tryptaminergic mechanisms in the elements of the behavioural syndrome evoked by tryptophan and a monoamine oxidase inhibitor [proceedings]. Br J Pharmacol. 1977 Mar;59(3):461P–461P. [PMC free article] [PubMed] [Google Scholar]
- Deakin J. F., Dashwood M. R. The differential neurochemical bases of the behaviours elicited by serotonergic agents and by the combination of a monoamine oxidase inhibitor and L-DOPA. Neuropharmacology. 1981 Feb;20(2):123–130. doi: 10.1016/0028-3908(81)90194-5. [DOI] [PubMed] [Google Scholar]
- Dourish C. T. Behavioural effects of acute and chronic beta-phenylethylamine administration in the rat: evidence for the involvement of 5-hydroxytryptamine. Neuropharmacology. 1981 Nov;20(11):1067–1072. doi: 10.1016/0028-3908(81)90098-8. [DOI] [PubMed] [Google Scholar]
- Dourish C. T., Boulton A. A. The effects of acute and chronic administration of beta-phenylethylamine on food intake and body weight in rats. Prog Neuropsychopharmacol. 1981;5(4):411–414. doi: 10.1016/0364-7722(81)90093-x. [DOI] [PubMed] [Google Scholar]
- Durden D. A., Philips S. R., Boulton A. A. Identification and distribution of beta-phenylethylamine in the rat. Can J Biochem. 1973 Jul;51(7):995–1002. doi: 10.1139/o73-129. [DOI] [PubMed] [Google Scholar]
- Durden D. A., Philips S. R. Kinetic measurements of the turnover rates of phenylethylamine and tryptamine in vivo in the rat brain. J Neurochem. 1980 Jun;34(6):1725–1732. doi: 10.1111/j.1471-4159.1980.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Fernando J. C., Lees A. J., Curzon G. Differential antagonism by neuroleptics of backward-walking and other behaviours caused by amphetamine at high dosage. Neuropharmacology. 1980 Jun;19(6):549–553. doi: 10.1016/0028-3908(80)90025-8. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971 Jun;18(6):1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Green A. R., Grahame-Smith D. G. (-)-Propranolol inhibits the behavioural responses of rats to increased 5-hydroxytryptamine in the central nervous system. Nature. 1976 Aug 12;262(5569):594–596. doi: 10.1038/262594a0. [DOI] [PubMed] [Google Scholar]
- Green A. R., Grahame-Smith D. G. The role of brain dopamine in the hyperactivity syndrome produced by increased 5-hydroxytryptamine synthesis in rats. Neuropharmacology. 1974 Nov;13(10-11):949–959. doi: 10.1016/0028-3908(74)90086-0. [DOI] [PubMed] [Google Scholar]
- Green A. R., Hall J. E., Rees A. R. A behavioural and biochemical study in rats of 5-hydroxytryptamine receptor agonists and antagonists, with observations on structure-activity requirements for the agonists. Br J Pharmacol. 1981 Jul;73(3):703–719. doi: 10.1111/j.1476-5381.1981.tb16806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. M. Some further observations of the effect of beta-phenethylamine on locomotor activity in mice. J Pharm Pharmacol. 1975 Apr;27(4):278–280. doi: 10.1111/j.2042-7158.1975.tb10700.x. [DOI] [PubMed] [Google Scholar]
- Jackson D. M. The effect of -phenethylamine upon spontaneous motor activity in mice: a dual effect on locomotor activity. J Pharm Pharmacol. 1972 May;24(5):383–389. doi: 10.1111/j.2042-7158.1972.tb09012.x. [DOI] [PubMed] [Google Scholar]
- Jackson D. M. The involvement of noradrenergic systems in the locomotor activity stimulation in mice produced by beta-phenethylamine. J Pharm Pharmacol. 1974 Aug;26(8):651–654. doi: 10.1111/j.2042-7158.1974.tb10684.x. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L. An animal behavior model for studying central serotonergic synapses. Life Sci. 1976 Sep 15;19(6):777–785. doi: 10.1016/0024-3205(76)90303-9. [DOI] [PubMed] [Google Scholar]
- Jones R. S. A comparison of the responses of cortical neurones to iontophoretically applied tryptamine and 5-hydroxytryptamine in the rat. Neuropharmacology. 1982 Mar;21(3):209–214. doi: 10.1016/0028-3908(82)90189-7. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Tollenaere J. P., Laduron P. M. Serotonergic component of neuroleptic receptors. Nature. 1978 Mar 9;272(5649):168–171. doi: 10.1038/272168a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. A., Curzon G. The contribution of tryptamine to the behavioural effects of l-Tryptophan in tranylcypromine-treated rats. Psychopharmacology (Berl) 1978 Apr 14;57(1):71–76. doi: 10.1007/BF00426960. [DOI] [PubMed] [Google Scholar]
- Mogilnicka E., Braestrup C. Noradrenergic influence on the stereotyped behaviour induced by amphetamine, phenethylamine and apomorphine. J Pharm Pharmacol. 1976 Mar;28(3):253–255. doi: 10.1111/j.2042-7158.1976.tb04143.x. [DOI] [PubMed] [Google Scholar]
- Moja E. A., Stoff D. M., Gillin J. C., Wyatt R. J. Dose-response effects of beta-phenylethylamine on stereotyped behavior in pargyline-pretreated rats. Biol Psychiatry. 1976 Dec;11(6):731–742. [PubMed] [Google Scholar]
- NAKAJIMA T., KAKIMOTO Y., SANO I. FORMATION OF BETA-PHENYLETHYLAMINE IN MAMMALIAN TISSUE AND ITS EFFECT ON MOTOR ACTIVITY IN THE MOUSE. J Pharmacol Exp Ther. 1964 Mar;143:319–325. [PubMed] [Google Scholar]
- Nelson D. L., Herbet A., Enjalbert A., Bockaert J., Hamon M. Serotonin-sensitive adenylate cyclase and [3H]serotonin binding sites in the CNS of the rat--I. Biochem Pharmacol. 1980 Sep 15;29(18):2445–2453. doi: 10.1016/0006-2952(80)90348-2. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. [3H]Mianserin: differential labeling of serotonin and histamine receptors in rat brain. J Pharmacol Exp Ther. 1981 Jan;216(1):142–148. [PubMed] [Google Scholar]
- Philips S. R., Rozdilsky B., Boulton A. A. Evidence for the presence of m-tyramine, p-tyramine, tryptamine, and phenylethylamine in the rat brain and several areas of the human brain. Biol Psychiatry. 1978 Feb;13(1):51–57. [PubMed] [Google Scholar]
- Potkin S. G., Karoum F., Chuang L. W., Cannon-Spoor H. E., Phillips I., Wyatt R. J. Phenylethylamine in paranoid chronic schizophrenia. Science. 1979 Oct 26;206(4417):470–471. doi: 10.1126/science.504988. [DOI] [PubMed] [Google Scholar]
- Randrup A., Munkvad I. DOPA and other naturally occurring substances as causes of stereotypy and rage in rats. Acta Psychiatr Scand Suppl. 1966;191:193–199. doi: 10.1111/j.1600-0447.1966.tb08804.x. [DOI] [PubMed] [Google Scholar]
- Randrup A., Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia. 1967;11(4):300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- Sabelli H. C., Mosnaim A. D. Phenylethylamine hypothesis of affective behavior. Am J Psychiatry. 1974 Jun;131(6):695–699. doi: 10.1176/ajp.131.6.695. [DOI] [PubMed] [Google Scholar]
- Sandler M., Reynolds G. P. Does phenylethylamine cause schizophrenia? Lancet. 1976 Jan 10;1(7950):70–71. doi: 10.1016/s0140-6736(76)90156-2. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S., Drust E. G., Connor J. D. Specificity of a rat behavioral model for serotonin receptor activation. J Pharmacol Exp Ther. 1978 Aug;206(2):339–347. [PubMed] [Google Scholar]
- Spano P. F., Biggio G., Casu M., Gessa G. L., Bareggi S. R., Govoni S., Trabucchi M. Interaction of metergoline with striatal dopamine system. Life Sci. 1978 Dec 11;23(24):2383–2391. doi: 10.1016/0024-3205(78)90296-5. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Weiss C., Gitter S. Blockade of 5-hydroxytryptamine receptors in the central nervous system by beta-adrenoceptor antagonists. Neuropharmacology. 1977 Apr;16(4):273–276. doi: 10.1016/0028-3908(77)90106-x. [DOI] [PubMed] [Google Scholar]
- Whitaker P. M., Seeman P. Selective labeling of serotonin receptors by d-[3H]lysergic acid diethylamide in calf caudate. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5783–5787. doi: 10.1073/pnas.75.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Boulton A. A. Metabolism distribution, and disappearance of injected beta-phenylethylamine in the rat. Can J Biochem. 1975 Jan;53(1):42–50. doi: 10.1139/o75-007. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Neff N. H. Beta-phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther. 1973 Nov;187(2):365–371. [PubMed] [Google Scholar]


