Abstract
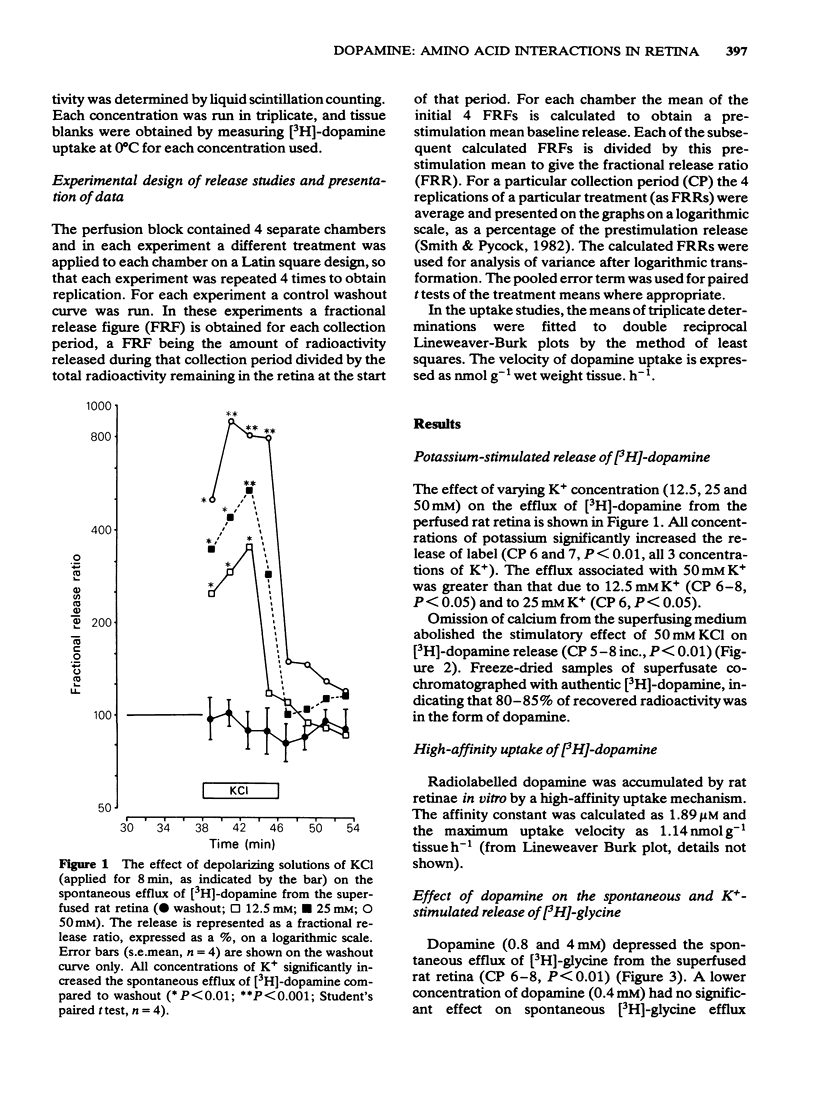
1 The dose-related, calcium-dependent, potassium-stimulated release of preloaded [3H]-dopamine from the superfused rat retina has been demonstrated.
2 A high-affinity uptake system for dopamine exists in rat retina in vitro; Km value was calculated as 1.89 μM, Vmax value as 1.4 nmol g-1 tissue h-1.
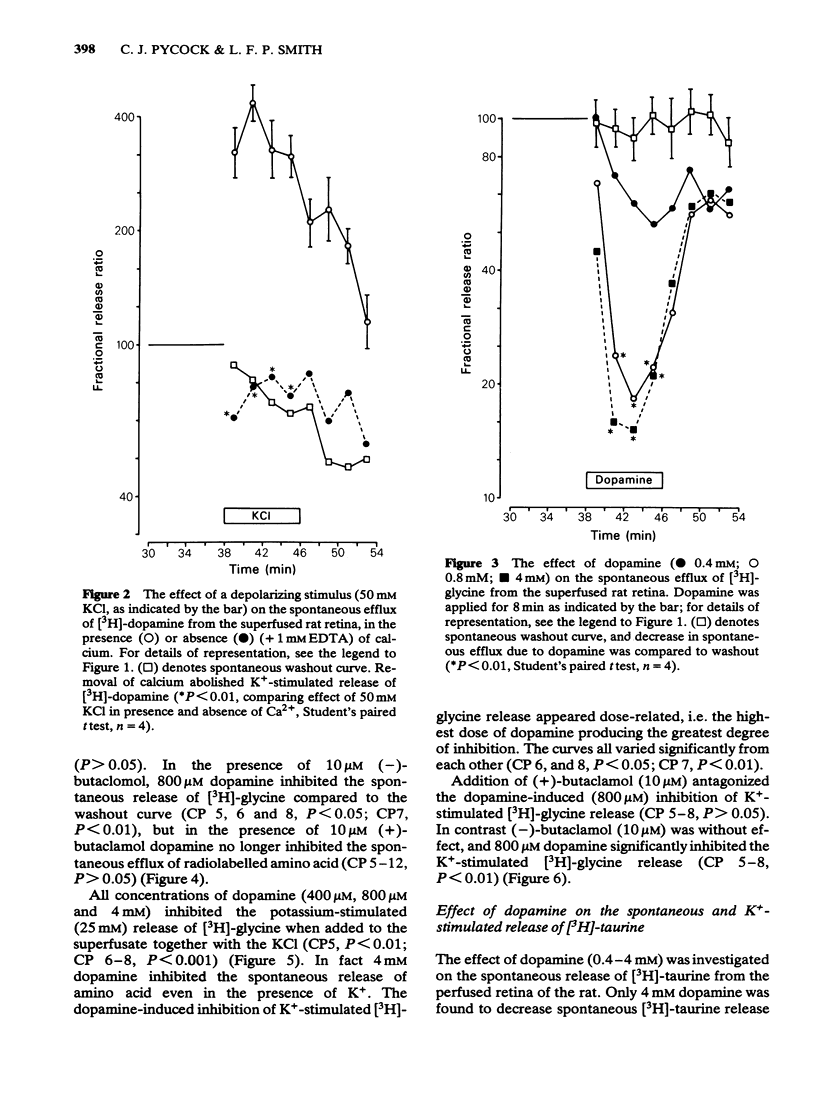
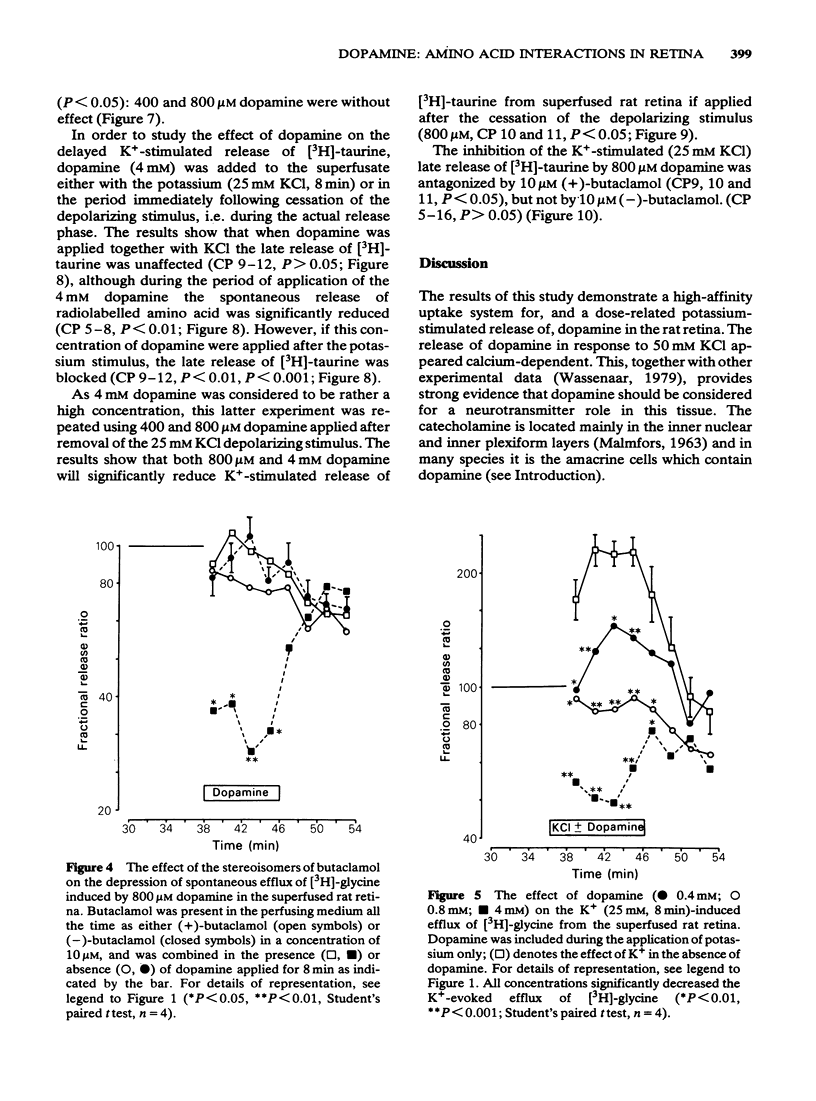
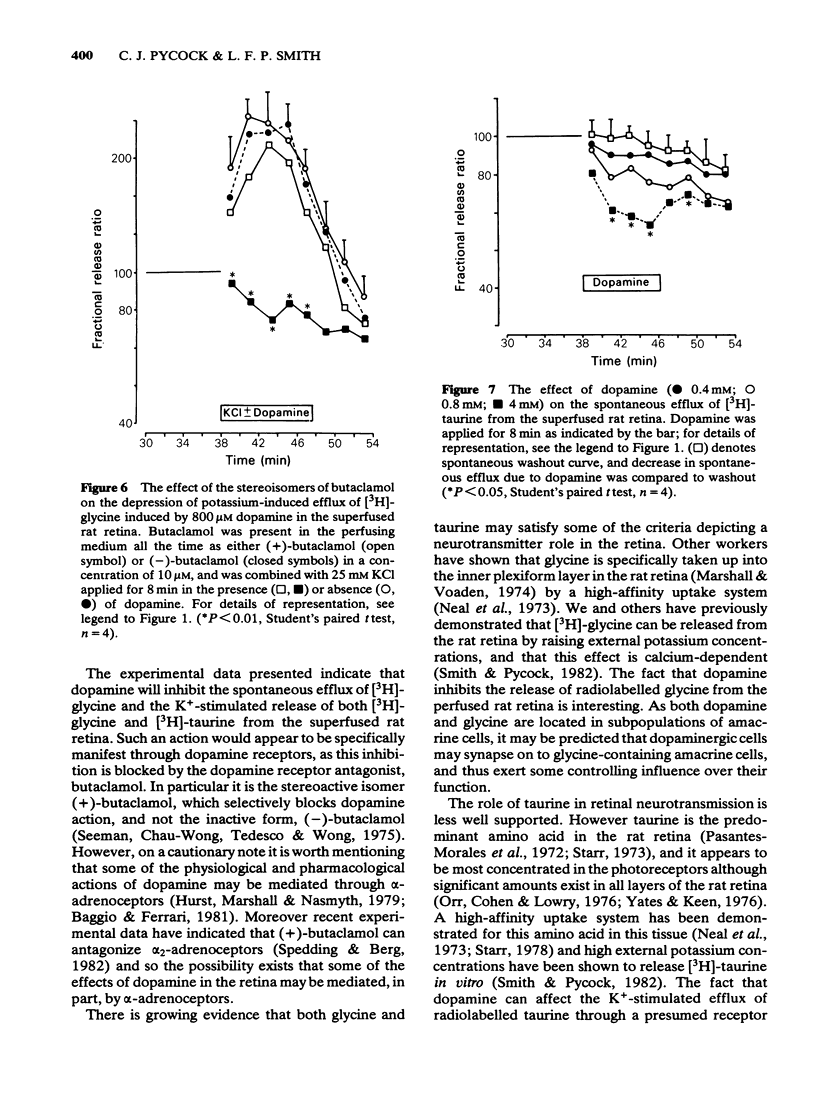
3 Dopamine (0.8 and 4 mM) inhibited the spontaneous release of [3H]-glycine from retina, and in the case of 0.8 mM dopamine this inhibitory effect was antagonized by 10 μM (+)-butaclamol but not by 10 μM (-)-butaclamol.
4 The potassium-evoked (25 mM) release of [3H]-glycine from rat retina was similarly inhibited by dopamine (0.4-4 mM) in a dose-related manner when added to the superfusate with the potassium. The effect of 0.8 mM dopamine was antagonized by 10 μM (+)-butaclamol but not by 10 μM (-)-butaclamol.
5 Dopamine (4 mM) significantly reduced the spontaneous release of [3H]-taurine from rat retina.
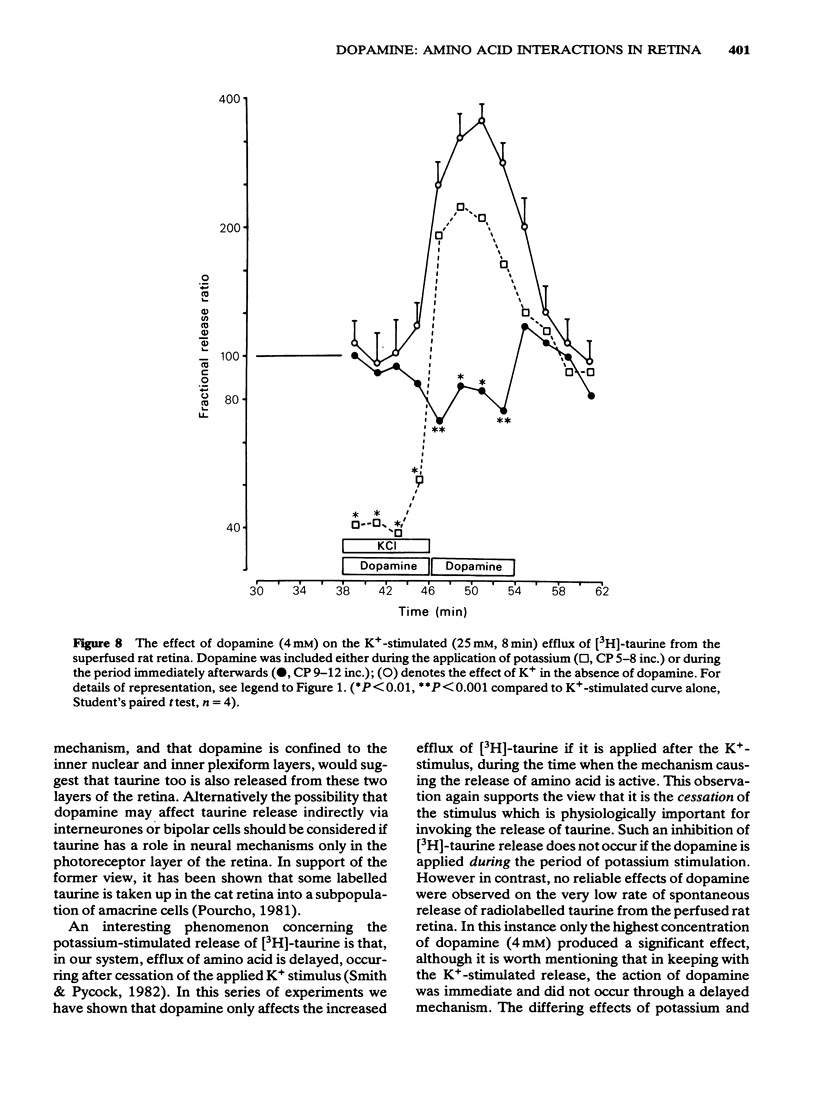
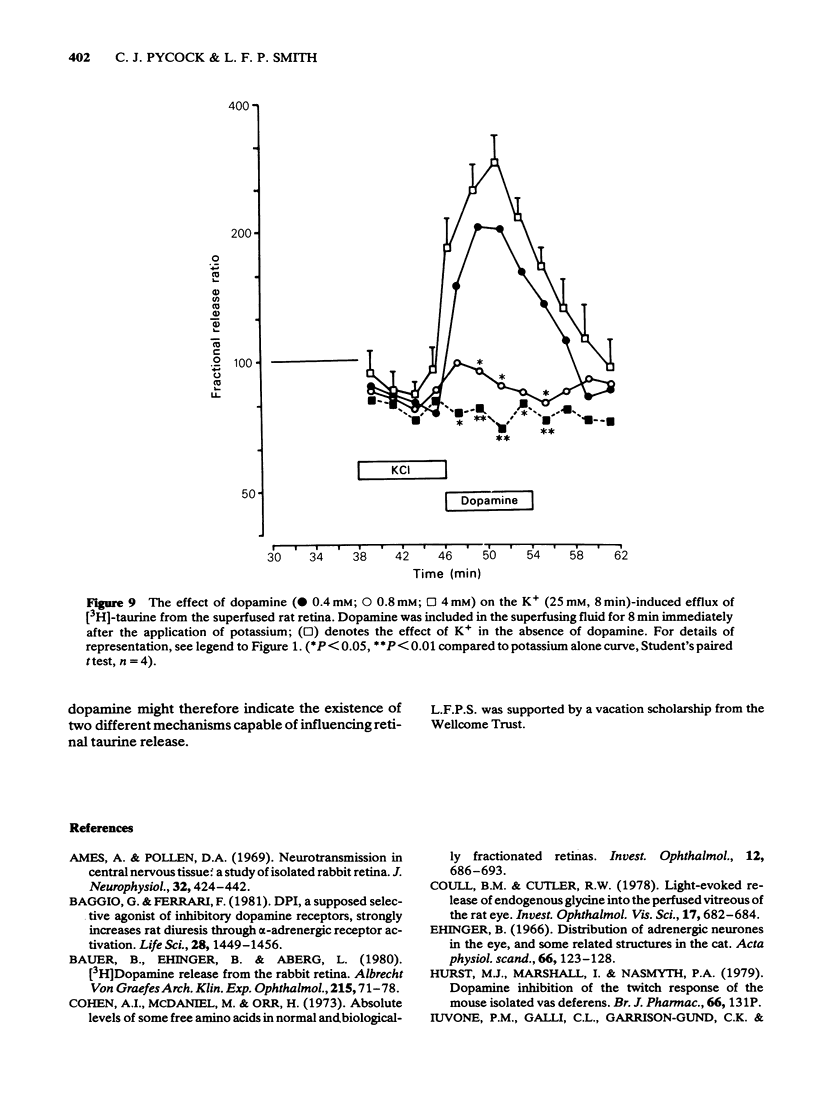
6 The potassium-stimulated (25 mM) release of [3H]-taurine occurred after the cessation of the depolarizing stimulus. This delayed release of [3H]-taurine was unaffected if dopamine was applied to the superfusate at the same time as the potassium, but it was significantly reduced if dopamine (0.8 and 4 mM) was applied after the depolarizing stimulus had been removed and during the actual amino acid release phase.
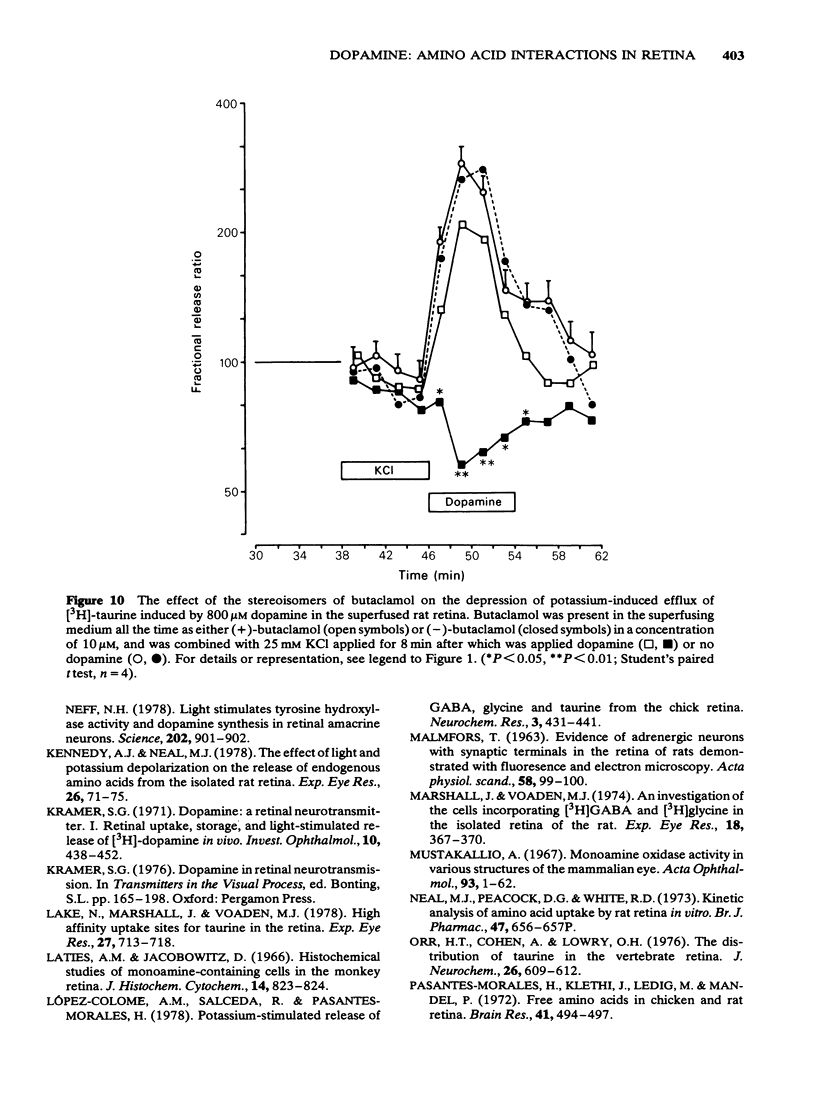
7 The inhibition of K+-stimulated (25 mM) delayed release of [3H]-taurine by applying dopamine (0.8 mM) after the depolarizing stimulus was blocked by 10 μM (+)-butaclamol but not by 10 μM (-)-butaclamol.
8 The results are discussed with respect to the possible neurotransmitter role for dopamine within the rat retina, and its possible interaction with glycine and taurine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Pollen D. A. Neurotransmission in central nervous tissue: a study of isolated rabbit retina. J Neurophysiol. 1969 May;32(3):424–442. doi: 10.1152/jn.1969.32.3.424. [DOI] [PubMed] [Google Scholar]
- Baggio G., Ferrari F. DPI, a supposed selective agonist of inhibitory dopamine receptors, strongly increases rat diuresis through alpha-adrenergic receptor activation. Life Sci. 1981 Mar 30;28(13):1449–1456. doi: 10.1016/0024-3205(81)90376-3. [DOI] [PubMed] [Google Scholar]
- Bauer B., Ehinger B., Aberg L. [3H]-dopamine release from the rabbit retina. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215(2):71–78. doi: 10.1007/BF00414464. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., McDaniel M., Orr H. Absolute levels of some free amino acids in normal and biologically fractionated retinas. Invest Ophthalmol. 1973 Sep;12(9):686–693. [PubMed] [Google Scholar]
- Coull B. M., Cutler R. W. Light-evoked release of endogenous glycine into the perfused vitreous of the intact rat eye. Invest Ophthalmol Vis Sci. 1978 Jul;17(7):682–684. [PubMed] [Google Scholar]
- Ehinger B. Distribution of adrenergic nerves in the eye and some related structures in the cat. Acta Physiol Scand. 1966 Jan-Feb;66(1):123–128. doi: 10.1111/j.1748-1716.1966.tb03176.x. [DOI] [PubMed] [Google Scholar]
- Hurst M. J., Marshall I., Nasmyth P. A. Dopamine inhibition of the twitch response of the mouse isolated vas deferens [proceedings]. Br J Pharmacol. 1979 May;66(1):131P–131P. [PMC free article] [PubMed] [Google Scholar]
- Iuvone P. M., Galli C. L., Garrison-Gund C. K., Neff N. H. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978 Nov 24;202(4370):901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Kennedy A. J., Neal M. J. The effect of light and potassium depolarization on the release of endogenous amino acids from the isolated rat retina. Exp Eye Res. 1978 Jan;26(1):71–75. doi: 10.1016/0014-4835(78)90153-7. [DOI] [PubMed] [Google Scholar]
- Kramer S. G. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol. 1971 Jun;10(6):438–452. [PubMed] [Google Scholar]
- Lake N., Marshall J., Voaden M. J. High affinity uptake sites for taurine in the retina. Exp Eye Res. 1978 Dec;27(6):713–8. doi: 10.1016/0014-4835(78)90040-4. [DOI] [PubMed] [Google Scholar]
- López-Colomé A. M., Salceda R., Pasantes-Morales H. Potassium-stimulated release of GABA, glycine, and taurine from the chick retina. Neurochem Res. 1978 Aug;3(4):431–441. doi: 10.1007/BF00966325. [DOI] [PubMed] [Google Scholar]
- MALMFORS T. Evidence of adrenergic neurons with synaptic terminals in the retina of rats demonstrated with fluorescence and electron microscopy. Acta Physiol Scand. 1963 May;58:99–100. doi: 10.1111/j.1748-1716.1963.tb02632.x. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. An investigation of the cells incorporating (3H)GABA and (3H)glycine in the isolated retina of the rat. Exp Eye Res. 1974 Apr;18(4):367–370. doi: 10.1016/0014-4835(74)90113-4. [DOI] [PubMed] [Google Scholar]
- Mustakallio A. Monoamine oxidase activity in the various structures of the mammalian eye. Histochemical staining and quantitative chemical study. Acta Ophthalmol (Copenh) 1967;(Suppl):1–62. [PubMed] [Google Scholar]
- Neal M. J., Peacock D. G., White R. D. Kinetic analysis of amino acid uptake by the rat retina in vitro. Br J Pharmacol. 1973 Mar;47(3):656P–657P. [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Cohen A. I., Lowry O. H. The distribution of taurine in the vertebrate retina. J Neurochem. 1976 Mar;26(3):609–611. doi: 10.1111/j.1471-4159.1976.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Klethi J., Ledig M., Mandel P. Free amino acids of chicken and rat retina. Brain Res. 1972 Jun 22;41(2):494–497. doi: 10.1016/0006-8993(72)90523-9. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G. [3H]taurine-accumulating neurons in the cat retina. Exp Eye Res. 1981 Jan;32(1):11–20. doi: 10.1016/s0014-4835(81)80034-6. [DOI] [PubMed] [Google Scholar]
- Sano Y., Yoshikawa H., Konishi M. Fluorescence microscopic observations on the dog retina. Arch Histol Jpn. 1968 Dec;30(1):75–81. doi: 10.1679/aohc1950.30.75. [DOI] [PubMed] [Google Scholar]
- Schaeffer J. M. Identification of dopamine receptors in the rat retina. Exp Eye Res. 1980 Apr;30(4):431–437. doi: 10.1016/0014-4835(80)90058-5. [DOI] [PubMed] [Google Scholar]
- Seeman P., Chau-Wong M., Tedesco J., Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. F., Pycock C. J. Potassium-stimulated release of radiolabelled taurine and glycine from the isolated rat retina. J Neurochem. 1982 Sep;39(3):653–658. doi: 10.1111/j.1471-4159.1982.tb07942.x. [DOI] [PubMed] [Google Scholar]
- Spano P. F., Govoni S., Hofmann M., Kumakura K., Trabucchi M. Physiological and pharmacological influences on dopaminergic receptors in the retina. Adv Biochem Psychopharmacol. 1977;16:307–310. [PubMed] [Google Scholar]
- Spedding M., Berg C. Stereospecific blockade of alpha 2-adrenoceptors by (+)-butaclamol: implications for the characterization of dopamine receptors. J Pharm Pharmacol. 1982 Jan;34(1):56–58. doi: 10.1111/j.2042-7158.1982.tb04681.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Neal M. J., Mitchell J. F. The effect of electrical stimulation and high potassium concentrations on the efflux of (3H) gamma-aminobutyric acid from brain slices. J Neurochem. 1969 Aug;16(8):1235–1244. doi: 10.1111/j.1471-4159.1969.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Starr M. S. Effect of dark adaptation on the GABA system in retina. Brain Res. 1973 Sep 14;59:331–338. doi: 10.1016/0006-8993(73)90271-0. [DOI] [PubMed] [Google Scholar]
- Starr M. S. Prospective neurotransmitters in vertebrate retina. Essays Neurochem Neuropharmacol. 1977;2:151–174. [PubMed] [Google Scholar]
- Starr M. S. Uptake of taurine by retina in different species. Brain Res. 1978 Aug 11;151(3):604–608. doi: 10.1016/0006-8993(78)91094-6. [DOI] [PubMed] [Google Scholar]
- Straschill M., Perwein J. The inhibition of retinal ganglion cells by catecholeamines and gamma-aminobutyric acid. Pflugers Arch. 1969;312(3):45–54. doi: 10.1007/BF00588530. [DOI] [PubMed] [Google Scholar]
- Yates R. A., Keen P. The distribution of free amino acids in subdivision of rat and frog retinae obtained by a new technique. Brain Res. 1976 Apr 30;107(1):117–126. doi: 10.1016/0006-8993(76)90099-8. [DOI] [PubMed] [Google Scholar]


