Abstract
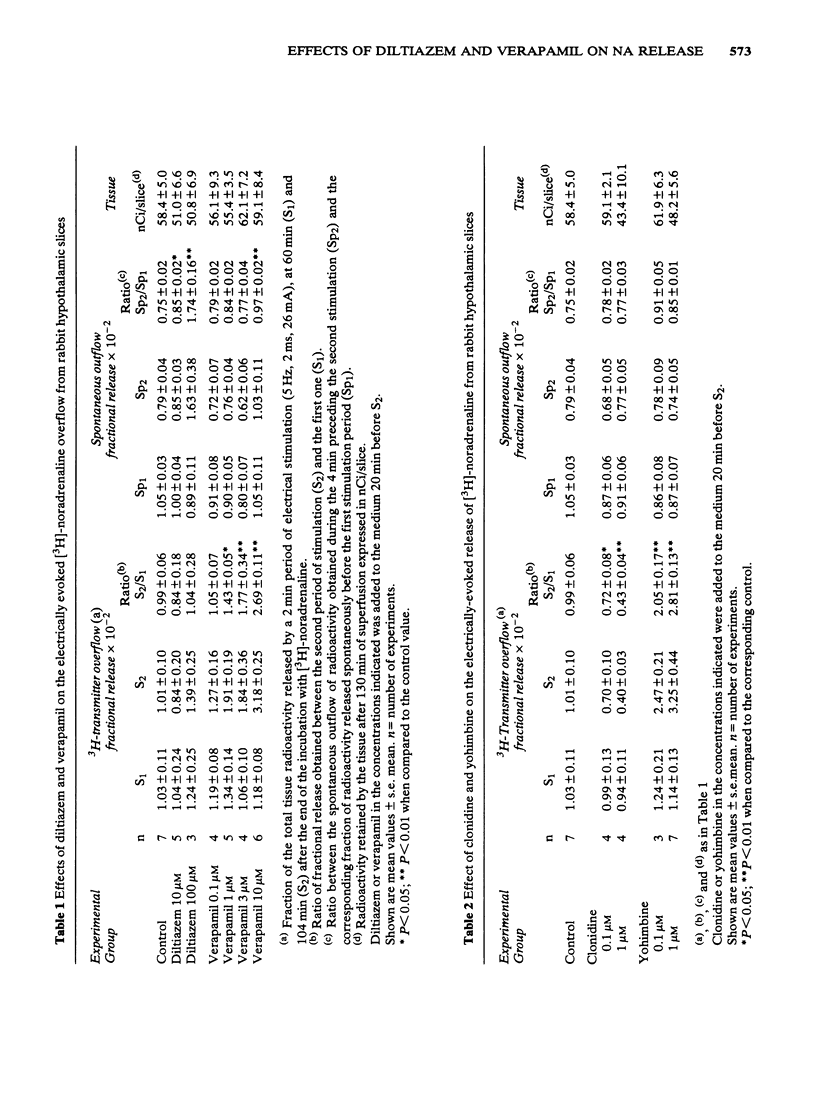
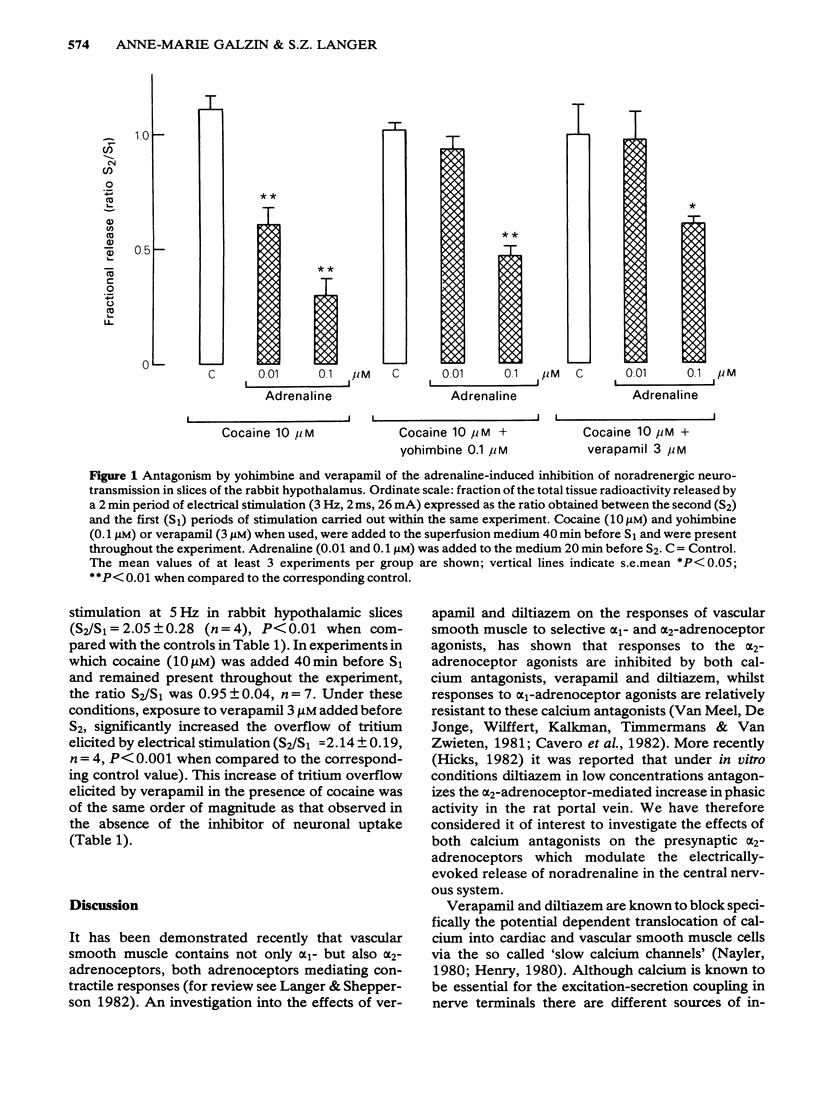
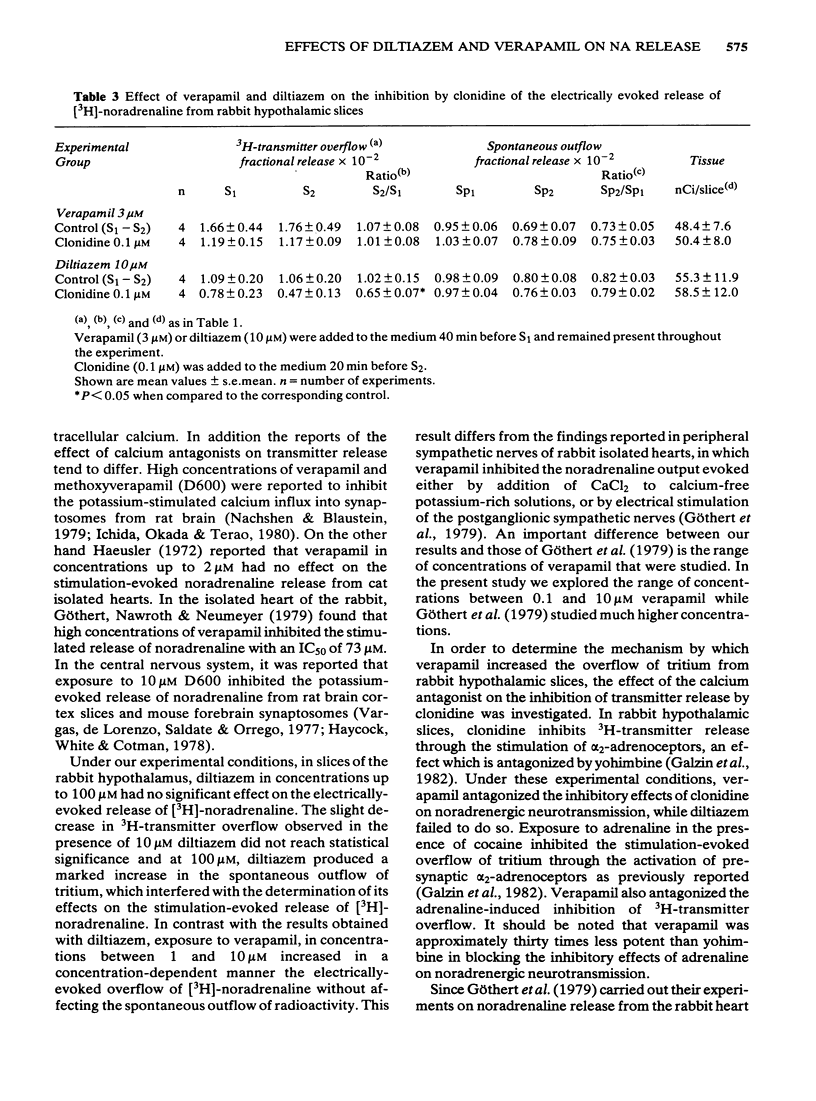
1 Rabbit hypothalamic slices prelabelled with [3H]-noradrenaline and superfused with Krebs solution were stimulated electrically at a frequency of 5 Hz. Exposure to verapamil (0.1 to 10 microM) significantly increased, in a concentration-dependent manner, the electrically-evoked overflow of tritium, without affecting the spontaneous outflow of radioactivity. 2 Exposure to diltiazem in concentrations up to 100 microM had no effect on the electrically evoked release of [3H]-noradrenaline, but increased the basal outflow of radioactivity at 10 and 100 microM. 3 The preferential alpha 2-adrenoceptor antagonist, yohimbine (0.1 microM) significantly antagonized the inhibitory effect of clonidine or adrenaline on [3H]-noradrenaline overflow elicited by electrical stimulation. Verapamil (3 microM) also antagonized this inhibitory effect of the alpha 2-adrenoceptor agonists on [3H]-noradrenaline release. In contrast to these results, exposure to diltiazem (10 microM) was ineffective in blocking the action of the alpha 2-adrenoceptor agonist. 4 These results suggest that the two Ca2+-antagonists verapamil and diltiazem differ in their ability to affect central noradrenergic neurotransmission. While verapamil is a relatively potent alpha 2-adrenoceptor antagonist, diltiazem is devoid of presynaptic alpha 2-adrenoceptor antagonist properties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Galzin A. M., Dubocovich M. L., Langer S. Z. Presynaptic inhibition by dopamine receptor agonists of noradrenergic neurotransmission in the rabbit hypothalamus. J Pharmacol Exp Ther. 1982 May;221(2):461–471. [PubMed] [Google Scholar]
- Göthert M., Nawroth P., Neumeyer H. Inhibitory effects of verapamil, prenylamine and D 600 on Ca2+-dependent noradrenaline release from the sympathetic nerves of isolated rabbit hearts. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(1):11–19. doi: 10.1007/BF00499869. [DOI] [PubMed] [Google Scholar]
- Haeusler G. Differential effect of verapamil on excitation-contraction coupling in smooth muscle and on excitation-secretion coupling in adrenergic nerve terminals. J Pharmacol Exp Ther. 1972 Mar;180(3):672–682. [PubMed] [Google Scholar]
- Haycock J. W., White W. F., Cotman C. W. Differences in alkaline earth stimulation of neurotransmitter release from isolated brain synaptosomes. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jan-Feb;301(3):175–179. doi: 10.1007/BF00507034. [DOI] [PubMed] [Google Scholar]
- Henry P. D. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am J Cardiol. 1980 Dec 1;46(6):1047–1058. doi: 10.1016/0002-9149(80)90366-5. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida S., Okada K., Terao M. Effect of verapamil on 45Ca uptake by synaptosomes. Jpn J Pharmacol. 1980 Apr;30(2):207–211. doi: 10.1254/jjp.30.207. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- Nayler W. G. Calcium antagonists. Eur Heart J. 1980 Jun;1(3):225–237. doi: 10.1093/oxfordjournals.eurheartj.a061123. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Thompson J. E., Jarrott B. The interaction of calcium antagonists (slow channel blockers) with myocardial alpha adrenoceptors. J Mol Cell Cardiol. 1982 Mar;14(3):185–188. doi: 10.1016/0022-2828(82)90118-3. [DOI] [PubMed] [Google Scholar]
- Pelayo F., Dubocovich M. L., Langer S. Z. Inhibition of neuronal uptake reduces the presynaptic effects of clonidine but not of alpha-methylnoradrenaline on the stimulation-evoked release of 3H-noradrenaline from rat occipital cortex slices. Eur J Pharmacol. 1980 Jun 13;64(2-3):143–155. doi: 10.1016/0014-2999(80)90037-0. [DOI] [PubMed] [Google Scholar]
- Van Meel J. C., De Jonge A., Kalkman H. O., Wilffert B., Timmermans P. B., Van Zwieten P. A. Vascular smooth muscle contraction initiated by postsynaptic alpha 2-adrenoceptor activation is induced by an influx of extracellular calcium. Eur J Pharmacol. 1981 Jan 16;69(2):205–208. doi: 10.1016/0014-2999(81)90415-5. [DOI] [PubMed] [Google Scholar]
- Vargas O., de Lorenzo M. D., Saldate M. C., Orrego F. Potassium-induced release of [3H] GABA and of [3H] noradrenaline from normal and reserpinized rat brain cortex slices, Differences in calcium-dependency, and in sensitivity to potassium ions. J Neurochem. 1977 Jan;28(1):165–170. doi: 10.1111/j.1471-4159.1977.tb07722.x. [DOI] [PubMed] [Google Scholar]


