Abstract
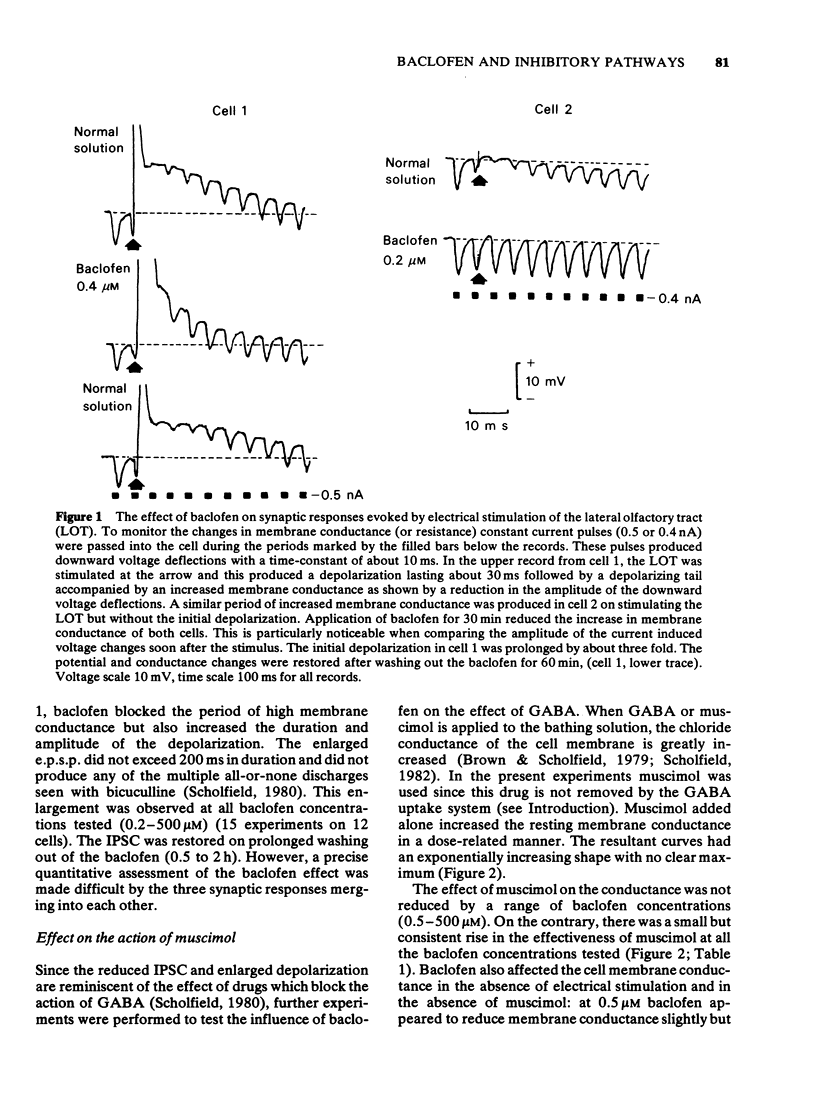
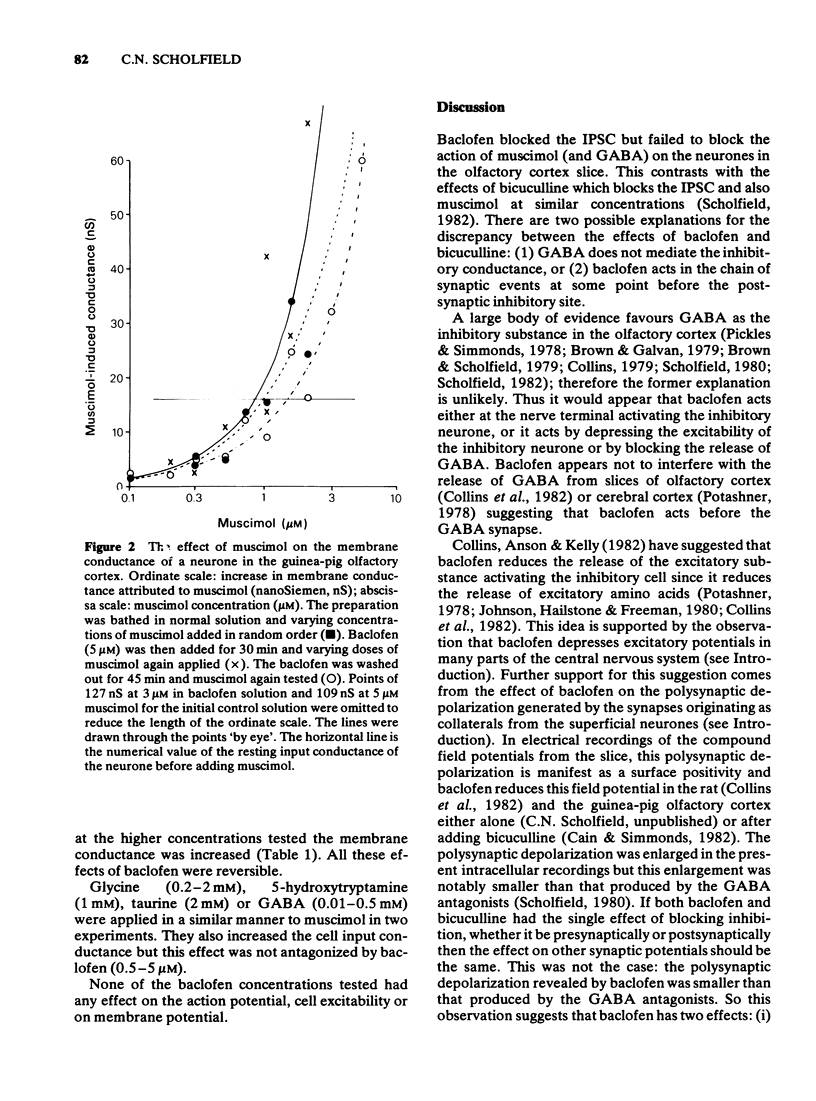
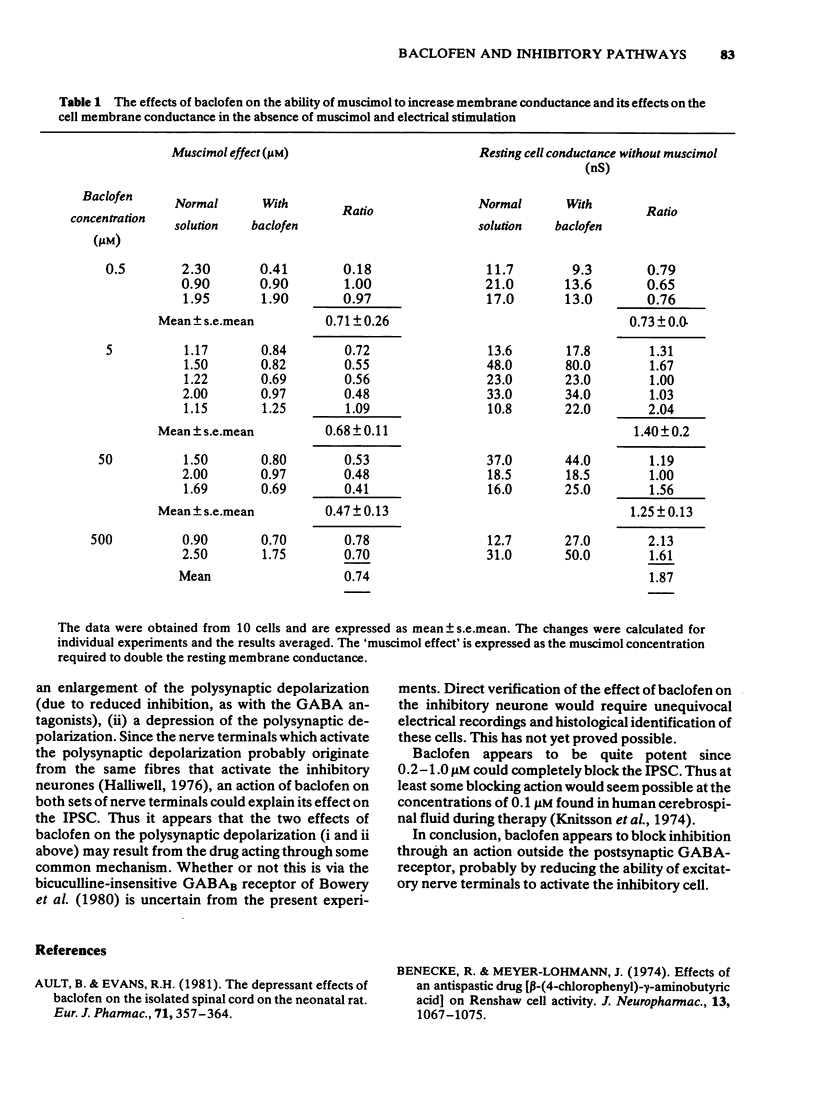
1 The olfactory cortex slice preparation from the guinea-pig brain was used to study the effects of baclofen on inhibition using intracellular recording. Stimulation of the lateral olfactory tract activities sequentially excitatory and inhibitory pathways. Inhibition is manifest as a period of increased membrane conductance (termed postsynaptic inhibitory conductance, IPSC). 2 Bath application of baclofen (0.2-500 muM) reversibly blocked the IPSC. Baclofen also produced a secondary increase in the amplitude and duration of the initial excitatory postsynaptic potential. 3 Baclofen (0.5-500 muM) slightly augmented the ability of bath-applied muscimol to increase the resting membrane conductance. Baclofen had no effect on cell excitability and membrane potential and no effect on the action of gamma-aminobutyric acid (GABA), noradrenaline, glycine, taurine or 5-hydroxytrypamine. 4 These results confirm previous suggestions that baclofen at low concentrations acts outside the GABA receptor mediating the IPSC perhaps by reducing the release of the excitatory transmitter activating the inhibitory interneurones.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Evans R. H. The depressant action of baclofen on the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1981 May 22;71(4):357–364. doi: 10.1016/0014-2999(81)90179-5. [DOI] [PubMed] [Google Scholar]
- Benecke R., Meyer-Lohmann J. Effects of an antispastic drug (beta-(4-chlorophenyl)-gamma-aminobutyric acid) on Renshaw cell activity. Neuropharmacology. 1974 Nov;13(10-11):1067–1075. doi: 10.1016/0028-3908(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Responses of the guinea-pig isolated olfactory cortex slice to gamma-aminobutyric acid recorded with extracellular electrodes. Br J Pharmacol. 1979 Feb;65(2):347–353. doi: 10.1111/j.1476-5381.1979.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Depolarization of neurones in the isolated olfactory cortex of the guinea-pig by gamma-aminobutyric acid. Br J Pharmacol. 1979 Feb;65(2):339–345. doi: 10.1111/j.1476-5381.1979.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. R., Simmonds M. A. Effects of baclofen on the olfactory cortex slice preparation. Neuropharmacology. 1982 Apr;21(4):371–373. doi: 10.1016/0028-3908(82)90103-4. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Anson J., Kelly E. P. Baclofen: effects on evoked field potentials and amino acid neurotransmitter release in the rat olfactory cortex slice. Brain Res. 1982 Apr 29;238(2):371–383. doi: 10.1016/0006-8993(82)90111-1. [DOI] [PubMed] [Google Scholar]
- Collins G. G. Evidence of a neurotransmitter role for aspartate and gamma-aminobutyric acid in the rat olfactory cortex. J Physiol. 1979 Jun;291:51–60. doi: 10.1113/jphysiol.1979.sp012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Bornstein J. C., Peet M. J. Selective effects of (-)-baclofen on spinal synaptic transmission in the cat. Exp Brain Res. 1981;42(2):158–170. doi: 10.1007/BF00236902. [DOI] [PubMed] [Google Scholar]
- Davies J. Selective depression of synaptic excitation in cat spinal neurones by baclofen: an iontophoretic study. Br J Pharmacol. 1981 Feb;72(2):373–384. doi: 10.1111/j.1476-5381.1981.tb09137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Hailstone M. H., Freeman C. G. Baclofen: stereoselective inhibition of excitant amino acid release. J Pharm Pharmacol. 1980 Mar;32(3):230–231. doi: 10.1111/j.2042-7158.1980.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Knutsson E., Lindblom U., Mårtensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci. 1974 Nov;23(3):473–484. doi: 10.1016/0022-510x(74)90163-4. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- Pickles H. G., Simmonds M. A. Field potentials, inhibition and the effect of pentobarbitone in the rat olfactory cortex slice. J Physiol. 1978 Feb;275:135–148. doi: 10.1113/jphysiol.1978.sp012181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release. Can J Physiol Pharmacol. 1978 Feb;56(1):150–154. doi: 10.1139/y78-019. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. A depolarizing inhibitory potential in neurones of the olfactory cortex in vitro. J Physiol. 1978 Feb;275:547–557. doi: 10.1113/jphysiol.1978.sp012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. Antagonism of gamma-aminobutyric acid and muscimol by picrotoxin, bicuculline, strychnine, bemegride, leptazol, D-tubocurarine and theophylline in the isolated olfactory cortex. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):274–280. doi: 10.1007/BF00501165. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Convulsants antagonise inhibition in the olfactory cortex slice. Naunyn Schmiedebergs Arch Pharmacol. 1980 Oct;314(1):29–36. doi: 10.1007/BF00498428. [DOI] [PubMed] [Google Scholar]


