Abstract
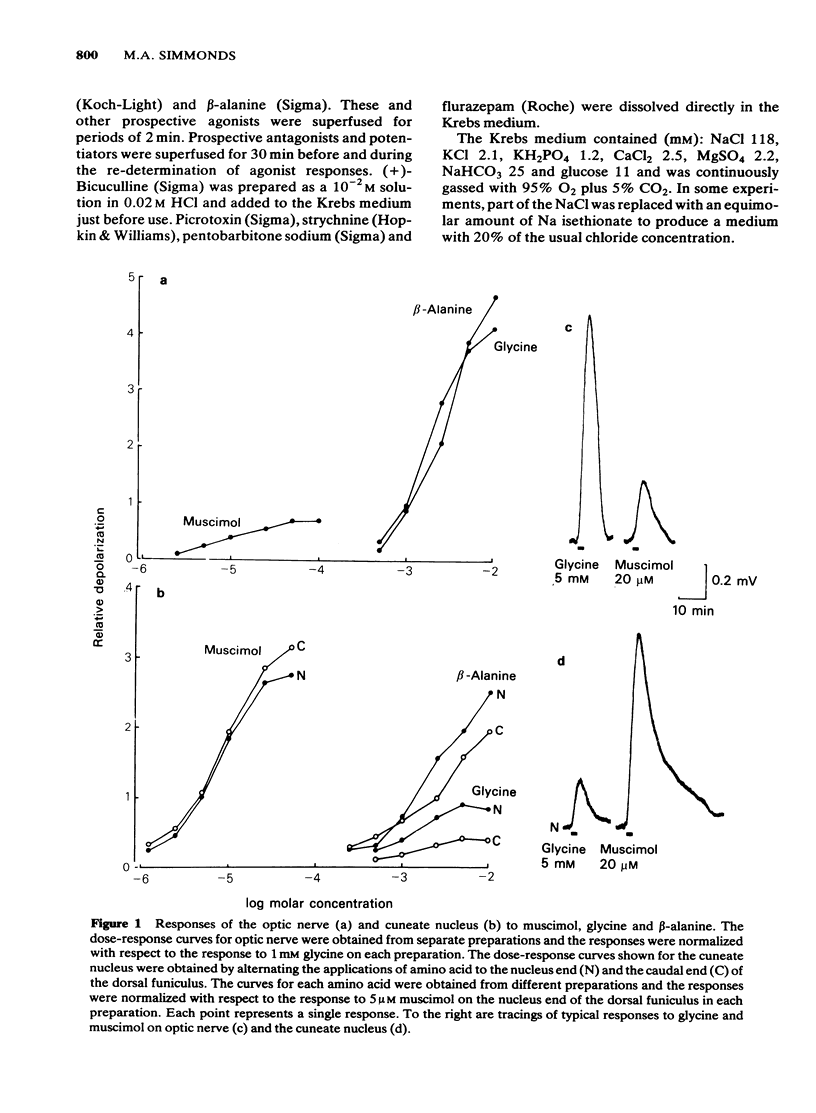
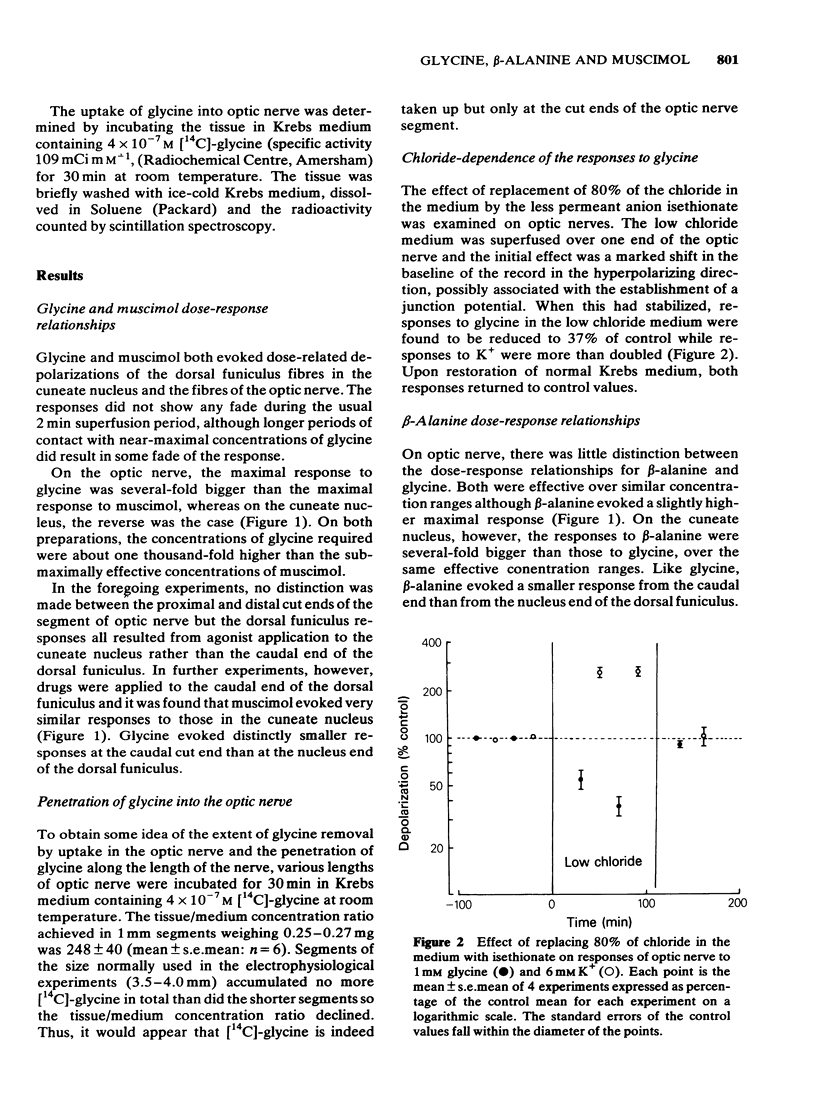
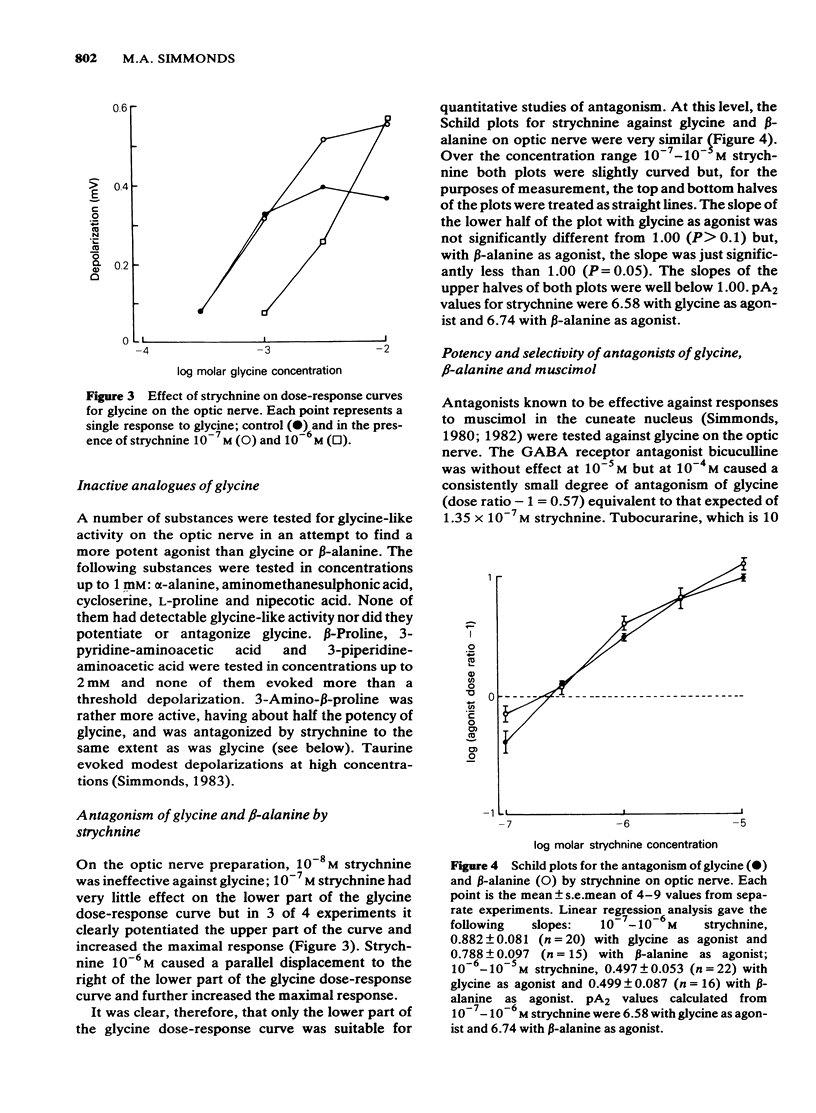
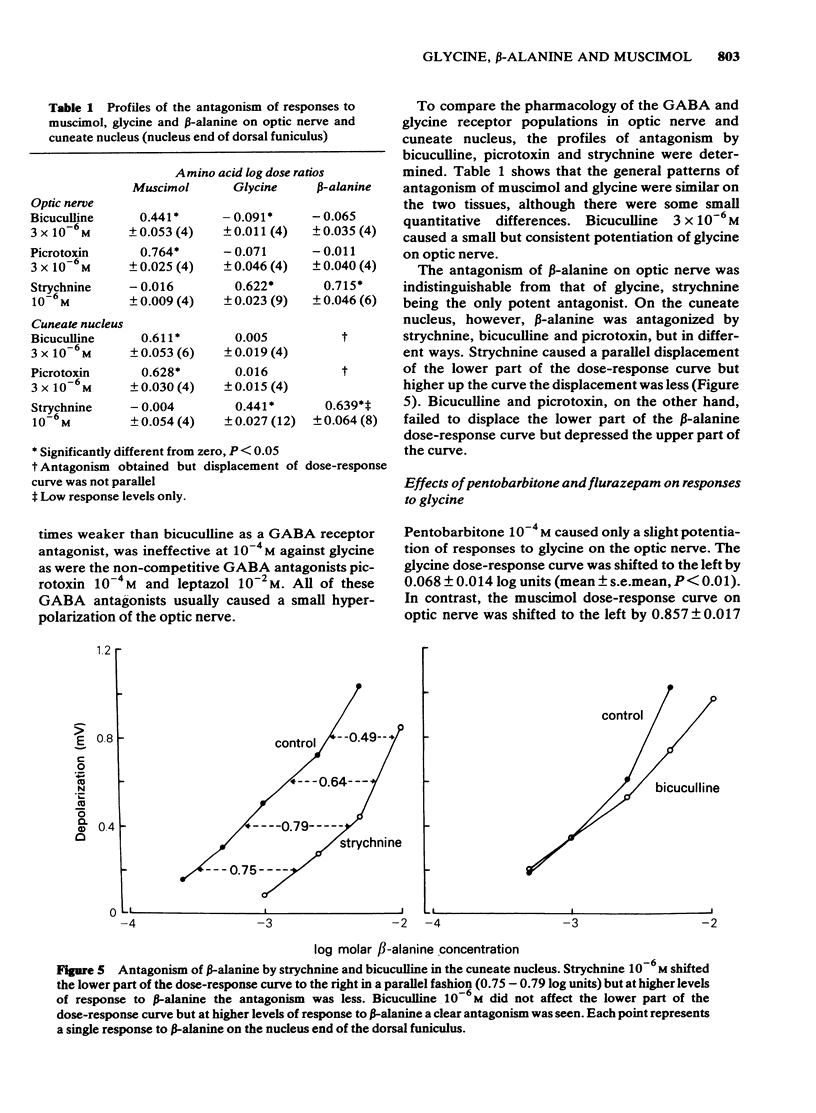
Concentration-dependent depolarizations were evoked by glycine and beta-alanine 5 X 10(-4)-10(-2)M and by the gamma-aminobutyric acid (GABA) analogue, muscimol 10(-6)-10(-4)M. The maximal response to glycine was several-fold higher than that to muscimol on optic nerve but the reverse was found on the dorsal funiculus fibres in the cuneate nucleus. beta-Alanine evoked a similar maximal response to glycine on optic nerve but a considerably higher maximum than glycine in the cuneate nucleus. Strychnine was 19.5 times more potent as a glycine antagonist (pA2 = 6.58) than as a muscimol antagonist. Bicuculline was 156 times more potent as a muscimol antagonist than as a glycine antagonist. Other antagonists of muscimol, i.e. tubocurarine, picrotoxin and leptazol, and potentiators of muscimol, i.e. pentobarbitone and flurazepam, had little or no effect on responses to glycine. Responses to beta-alanine had pharmacological properties compatible with a mixed action on both GABA and glycine receptors. The rat isolated optic nerve appears to be a useful preparation for studying the pharmacology of the neuronal glycine receptor plus chloride ionophore complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. D., Evans R. H., Johnston G. A. gamma-Aminobutyric acid agonists: an in vitro comparison between depression of spinal synaptic activity and depolarization of spinal root fibres in the rat. Br J Pharmacol. 1980 Dec;70(4):609–615. doi: 10.1111/j.1476-5381.1980.tb09779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978 Nov 3;156(1):187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- Burton H., Loewy A. D. Projections to the spinal cord from medullary somatosensory relay nuclei. J Comp Neurol. 1977 Jun 15;173(4):773–792. doi: 10.1002/cne.901730408. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., McLennan H. Antagonism between bicuculline and GABA in the cat brain. Brain Res. 1971 Oct 8;33(1):57–73. doi: 10.1016/0006-8993(71)90305-2. [DOI] [PubMed] [Google Scholar]
- Hill R. G., Simmonds M. A., Straughan D. W. Antagonism of gamma-aminobutyric acid and glycine by convulsants in the cuneate nucleus of cat. Br J Pharmacol. 1976 Jan;56(1):9–19. doi: 10.1111/j.1476-5381.1976.tb06952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. P., Beek D. The measurement of antagonist potency and the importance of selective inhibition of agonist uptake processes. J Pharmacol Exp Ther. 1981 Oct;219(1):112–120. [PubMed] [Google Scholar]
- Levy R. A. The role of GABA in primary afferent depolarization. Prog Neurobiol. 1977;9(4):211–267. doi: 10.1016/0301-0082(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Pickles H. G. Presynaptic gamma-aminobutyric acid responses in the olfactory cortex. Br J Pharmacol. 1979 Feb;65(2):223–228. doi: 10.1111/j.1476-5381.1979.tb07822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles H. G., Simmonds M. A. Antagonism by penicillin of gamma-aminobutyric acid depolarizations at presynaptic sites in rat olfactory cortex and cuneate nucleus in vitro. Neuropharmacology. 1980 Jan;19(1):35–38. doi: 10.1016/0028-3908(80)90163-x. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Classification of some GABA antagonists with regard to site of action and potency in slices of rat cuneate nucleus. Eur J Pharmacol. 1982 Jun 4;80(4):347–358. doi: 10.1016/0014-2999(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Distinction between the effects of barbiturates, benzodiazepines and phenytoin on responses to gamma-aminobutyric acid receptor activation and antagonism by bicuculline and picrotoxin. Br J Pharmacol. 1981 Jul;73(3):739–747. doi: 10.1111/j.1476-5381.1981.tb16810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M. A. Evidence that bicuculline and picrotoxin act at separate sites to antagonize gamma-aminobutyric acid in rat cuneate nucleus. Neuropharmacology. 1980 Jan;19(1):39–45. doi: 10.1016/0028-3908(80)90164-1. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Presynaptic actions of gamma-aminobutyric acid and some antagonists in a slice preparation of cuneate nucleus. Br J Pharmacol. 1978 Jul;63(3):495–502. doi: 10.1111/j.1476-5381.1978.tb07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


