Abstract
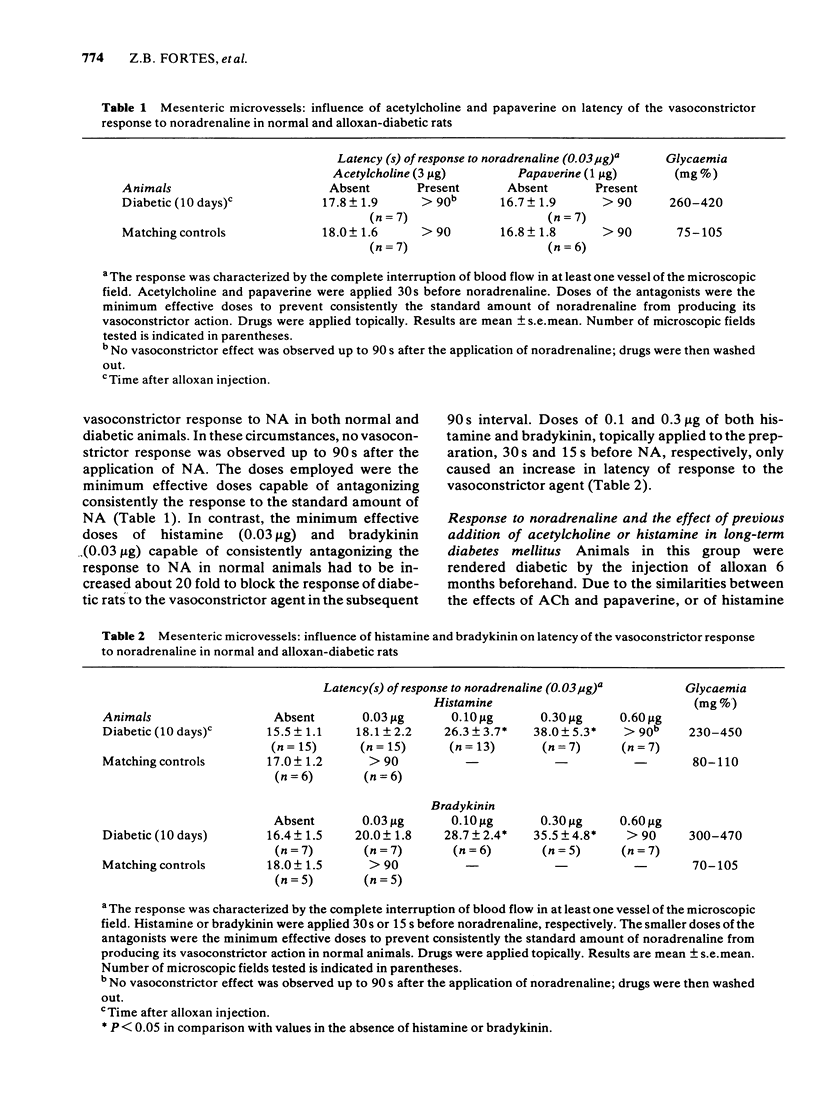
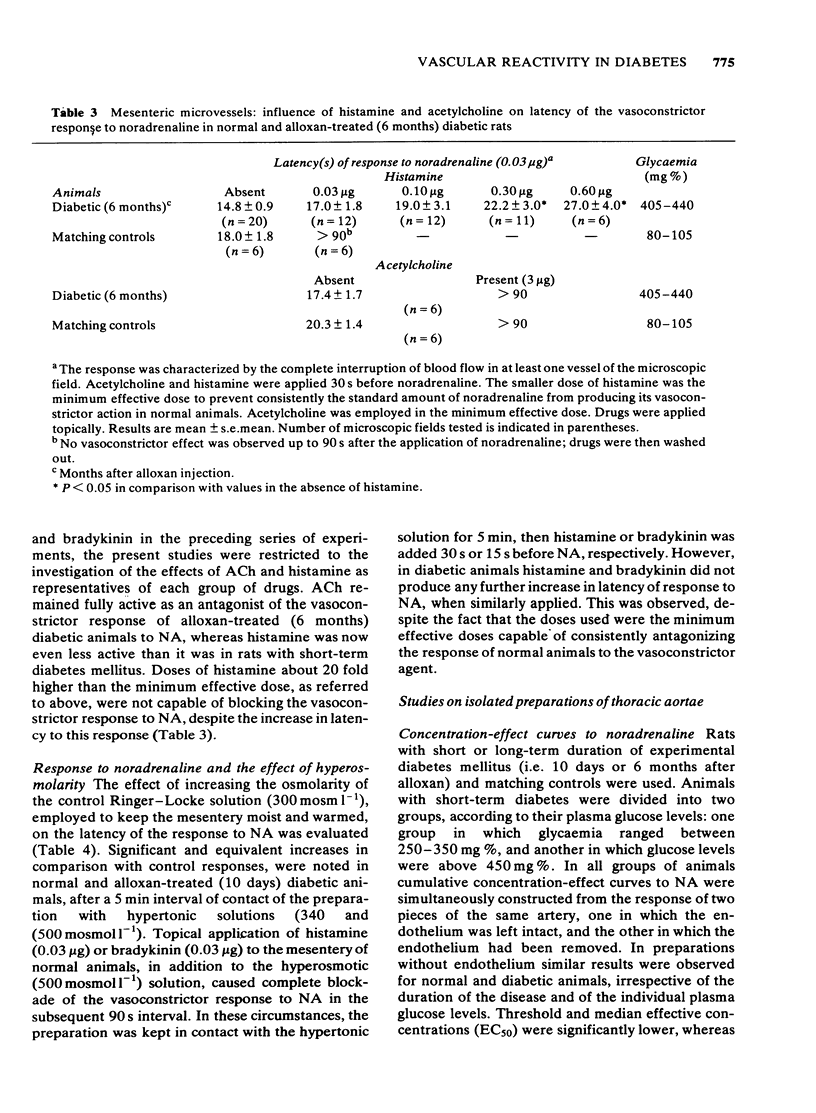
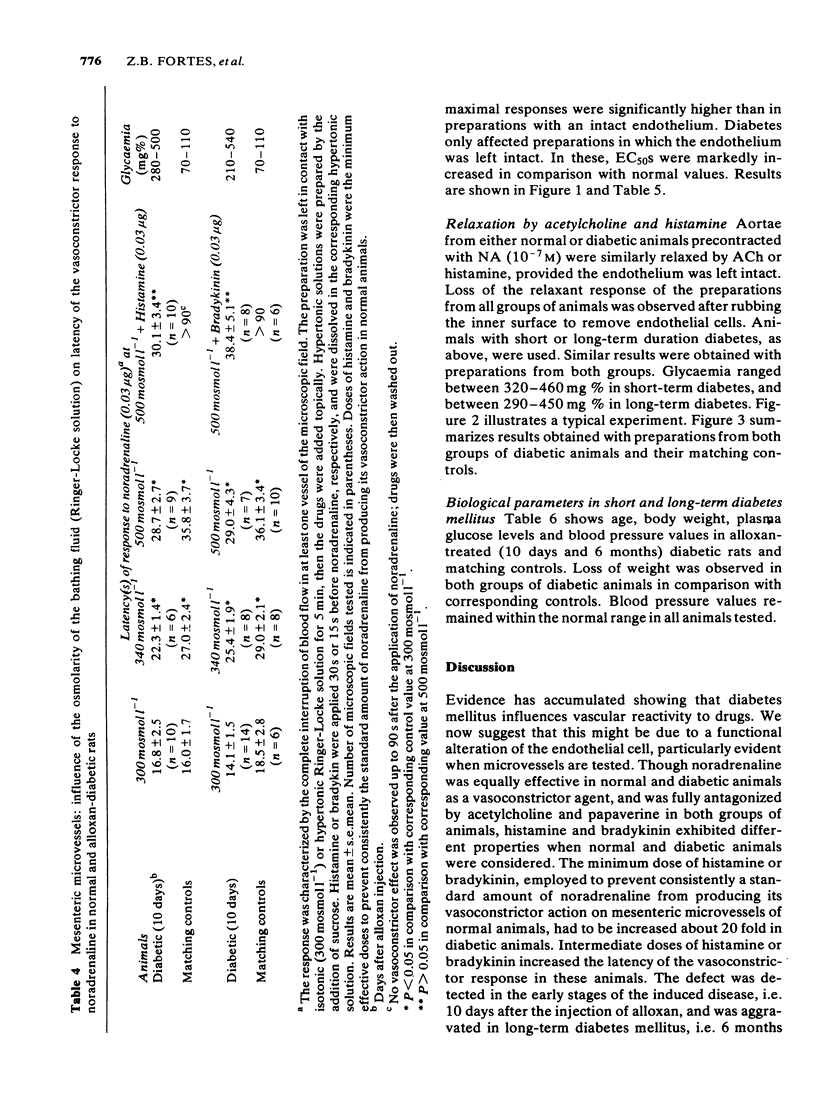
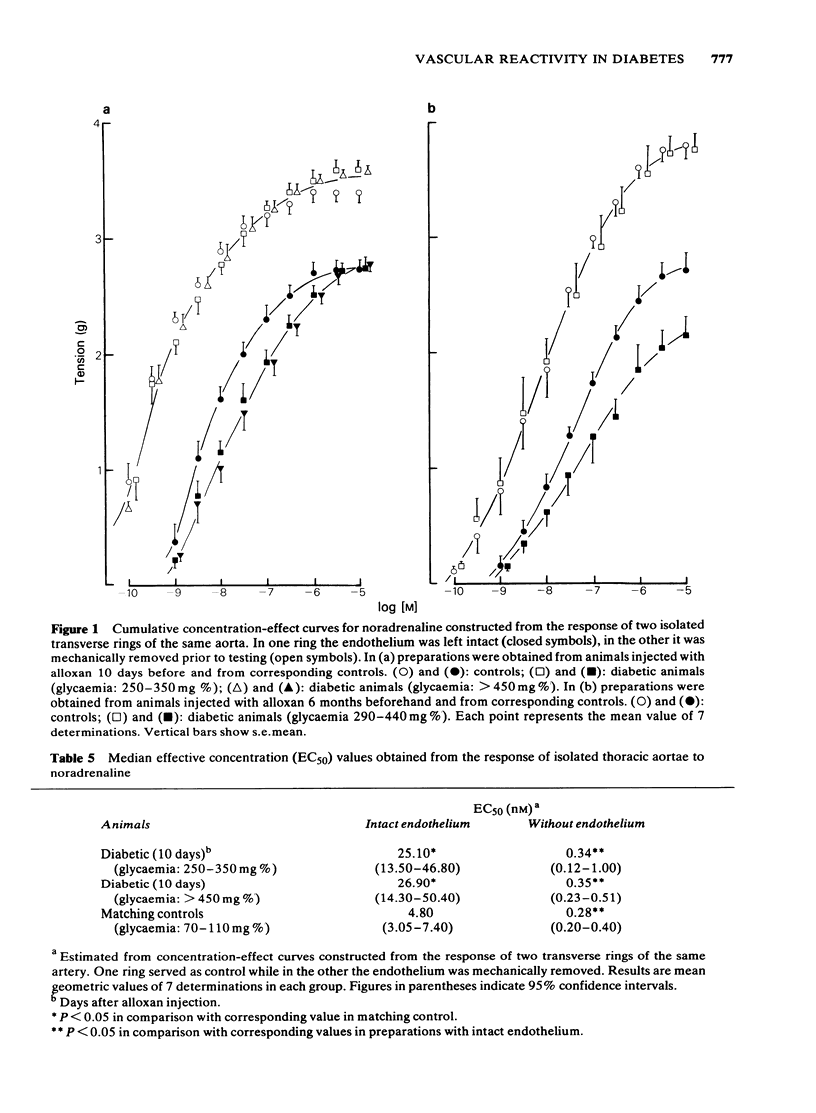
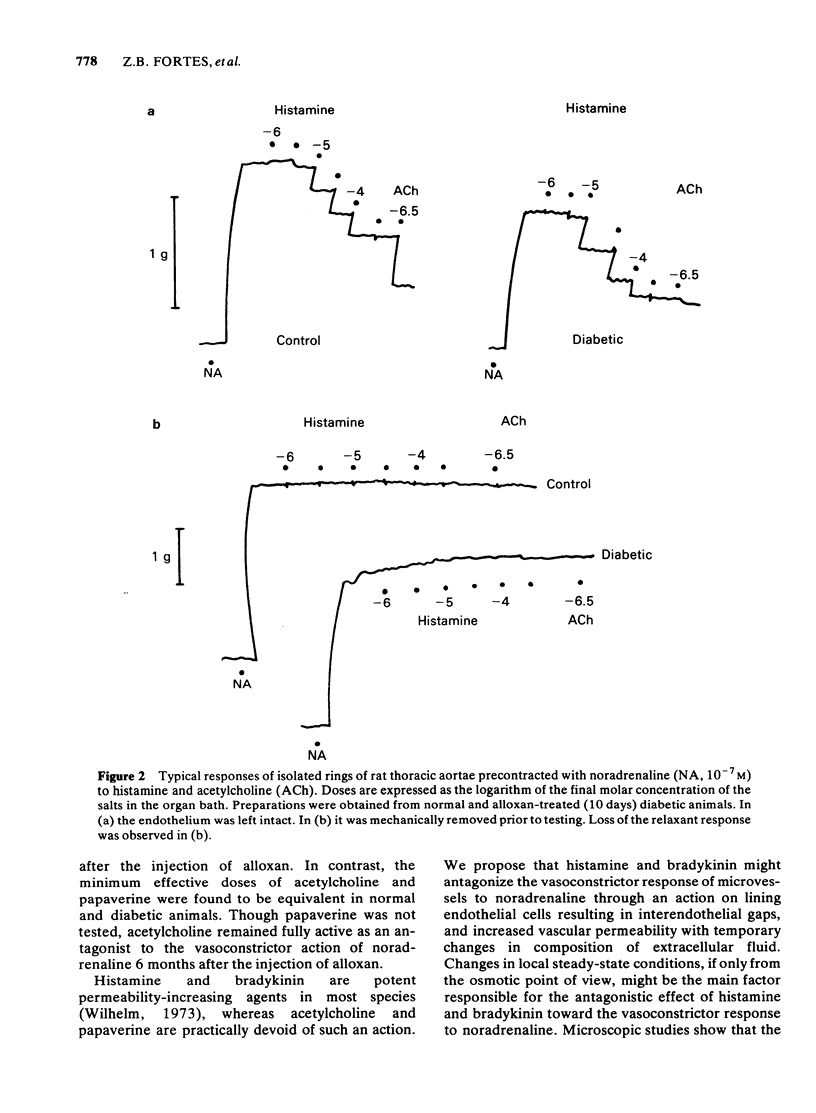
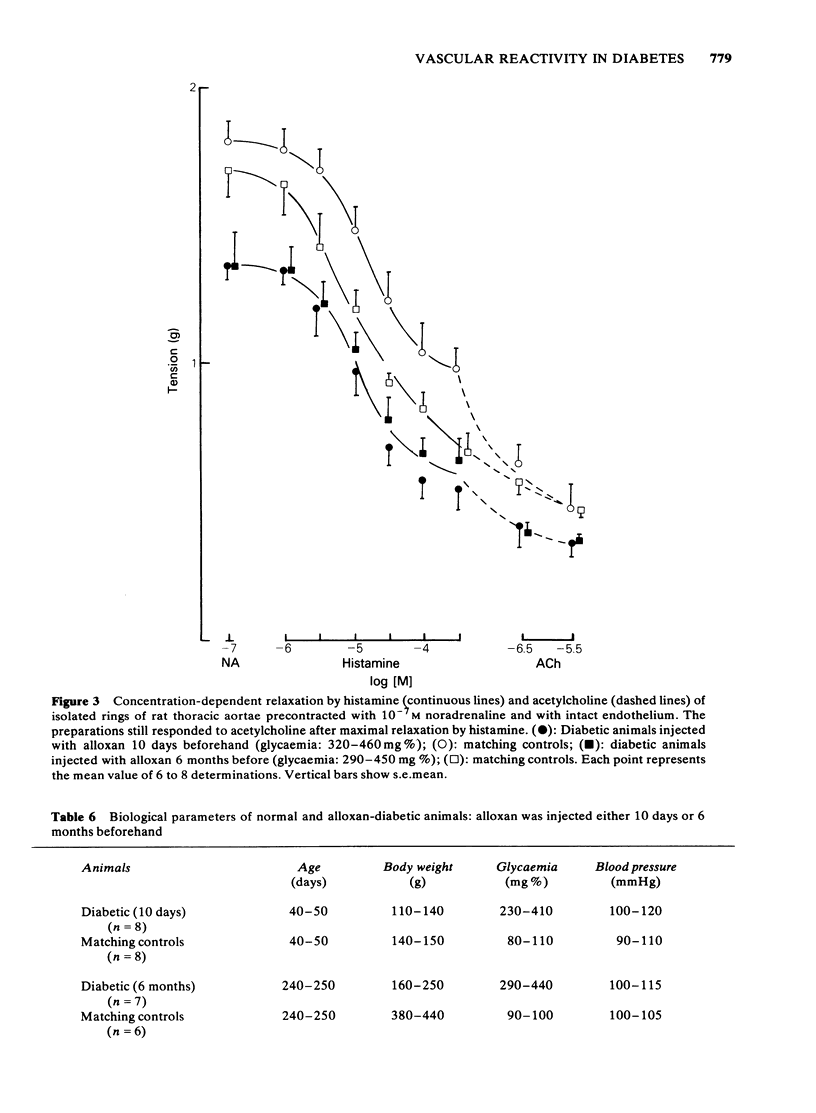
The response to vasoactive agents of microvessels in situ and large arteries in vitro was compared in normal and alloxan-diabetic rats. Noradrenaline was equally effective in evoking a constrictor response of mesenteric microvessels in normal and diabetic animals. The constrictor response to a standard amount of noradrenaline in such vessels was fully antagonized by acetylcholine or papaverine, the minimum effective doses being equivalent in normal and diabetic animals. In contrast, the minimum doses of histamine or bradykinin, effective in normal animals, had to be increased about 20 fold to be active in diabetic animals. Increased osmolarity of extracellular fluid caused a significant and equivalent increase in latency of the vasoconstrictor response of microvessels to noradrenaline in normal and diabetic animals. Concentration-effect curves, constructed from the response of isolated aortae to noradrenaline, were similar in normal and diabetic animals, provided the endothelium was removed. Diabetes only affected preparations in which the endothelium was left intact. In these, the median effective concentrations of noradrenaline were greatly increased in comparison with normal values. Precontracted aortae from normal and diabetic animals were equally relaxed by acetylcholine and histamine, provided the endothelium was left intact. Loss of the relaxant response of the preparations in all groups of animals was observed following removal of endothelial cells. It is suggested that different mechanisms may be involved in the effects of vasodilator agents on large arteries in vitro or small vessels in situ. Histamine and bradykinin which are potent permeability-increasing factors, may antagonize the vasoconstrictor response of microvessels to noradrenaline through an action on endothelial cells with increased vascular permeability and temporary changes in composition of extracellular fluid. The reactive process of endothelial cells to permeability factors was affected by diabetes mellitus. However, the response of microvessels to acetylcholine and papaverine which are devoid of permeability-increasing properties, was not influenced by diabetes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chand N., Altura B. M. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung vascular diseases. Science. 1981 Sep 18;213(4514):1376–1379. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- Duling B. R., Staples E. Microvascular effects of hypertonic solutions in the hamster. Microvasc Res. 1976 Jan;11(1):51–56. doi: 10.1016/0026-2862(76)90076-5. [DOI] [PubMed] [Google Scholar]
- Fortes Z. B., Garcia Leme J., Scivoletto R. Influence of diabetes on the reactivity of mesenteric microvessels to histamine, bradykinin and acetylcholine. Br J Pharmacol. 1983 Jan;78(1):39–48. doi: 10.1111/j.1476-5381.1983.tb09360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Leme J., Böhm G. M., Migliorini R. H., de Souza M. Z. Possible participation of insulin in the control of vascular permeability. Eur J Pharmacol. 1974 Dec;29(2):298–306. doi: 10.1016/0014-2999(74)90030-2. [DOI] [PubMed] [Google Scholar]
- Gray S. D. Effect of hypertonicity on vascular dimensions in skeletal muscle. Microvasc Res. 1971 Jan;3(1):117–124. doi: 10.1016/0026-2862(71)90016-1. [DOI] [PubMed] [Google Scholar]
- King E. J., Garner R. J. The Colorimetric Determination of Glucose. J Clin Pathol. 1947 Nov;1(1):30–33. doi: 10.1136/jcp.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leme J. G., Hamamura L., Migliorini R. H., Leite M. P. Influence of diabetes upon the inflammatory response of the rat. A pharmacological analysis. Eur J Pharmacol. 1973 Jul;23(1):74–81. doi: 10.1016/0014-2999(73)90246-x. [DOI] [PubMed] [Google Scholar]
- Leme J. G. Regulatory mechanisms in inflammation: new aspects of autopharmacology. Gen Pharmacol. 1981;12(1):15–24. doi: 10.1016/0306-3623(81)90022-7. [DOI] [PubMed] [Google Scholar]
- Llorach M. A., Böhm G. M., Leme J. G. Decreased vascular reactions to permeability factors in experimental diabetes. Br J Exp Pathol. 1976 Dec;57(6):747–754. [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. A., Shepherd J. T. Hyperosmolarity: effects on nerves and smooth muscle of cutaneous veins. Am J Physiol. 1976 Jul;231(1):141–147. doi: 10.1152/ajplegacy.1976.231.1.141. [DOI] [PubMed] [Google Scholar]
- Osterby R., Gundersen H. J., Christensen N. J. The acute effect of insulin on capillary endothelial cells. Diabetes. 1978 Jul;27(7):745–749. doi: 10.2337/diab.27.7.745. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J., Leusen I. Vascular endothelium and the relaxation effect of histamine on aorta of the rat. Arch Int Pharmacodyn Ther. 1982 Apr;256(2):329–330. [PubMed] [Google Scholar]
- Wahl M., Kuschinsky W., Bosse O., Thurau K. Dependency of pial arterial and arteriolar diameter on perivascular osmolarity in the cat. A microapplication study. Circ Res. 1973 Feb;32(2):162–169. doi: 10.1161/01.res.32.2.162. [DOI] [PubMed] [Google Scholar]
- Williams J. R., Harrison T. R., Grollman A. A SIMPLE METHOD FOR DETERMINING THE SYSTOLIC BLOOD PRESSURE OF THE UNANESTHETIZED RAT. J Clin Invest. 1939 May;18(3):373–376. doi: 10.1172/JCI101051. [DOI] [PMC free article] [PubMed] [Google Scholar]


