Abstract
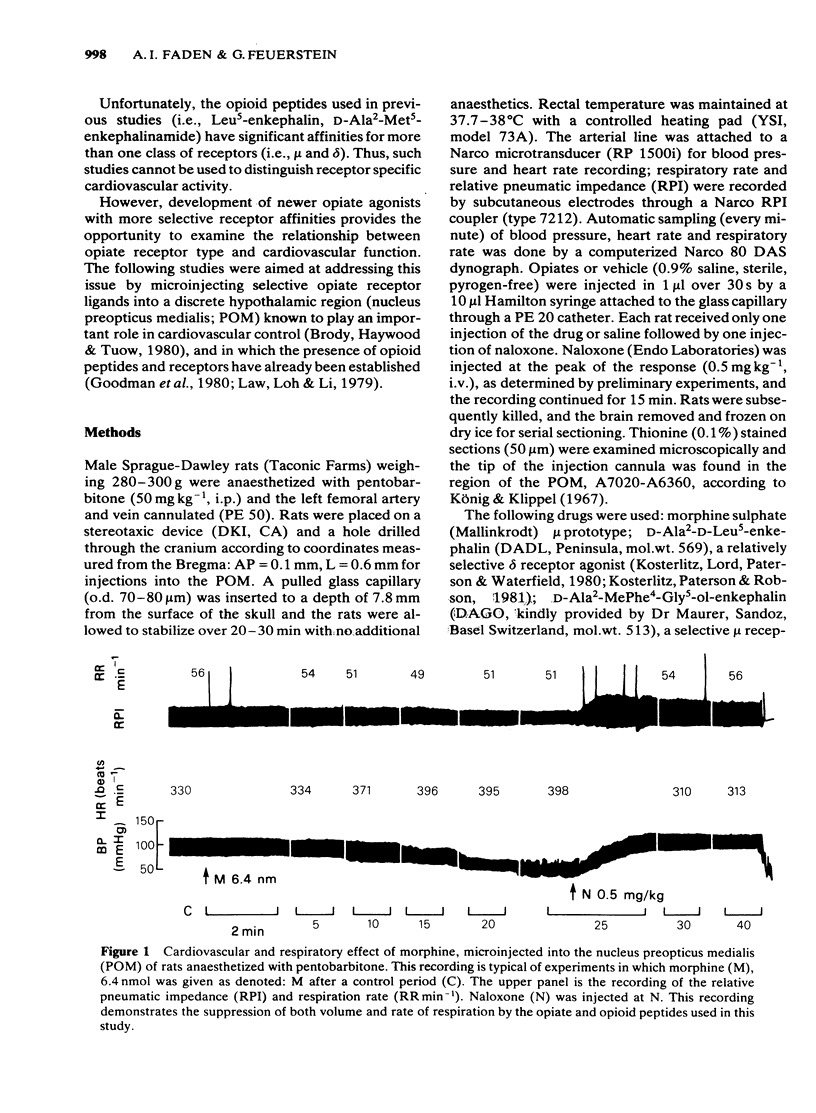
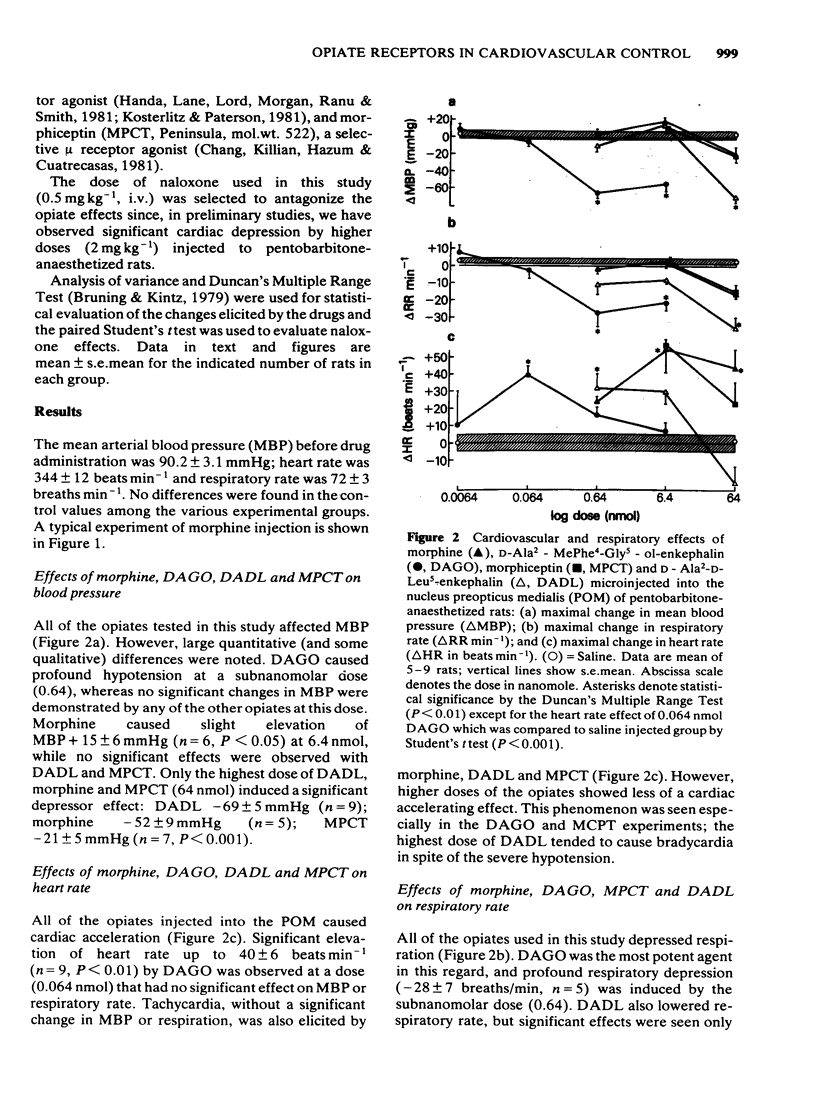
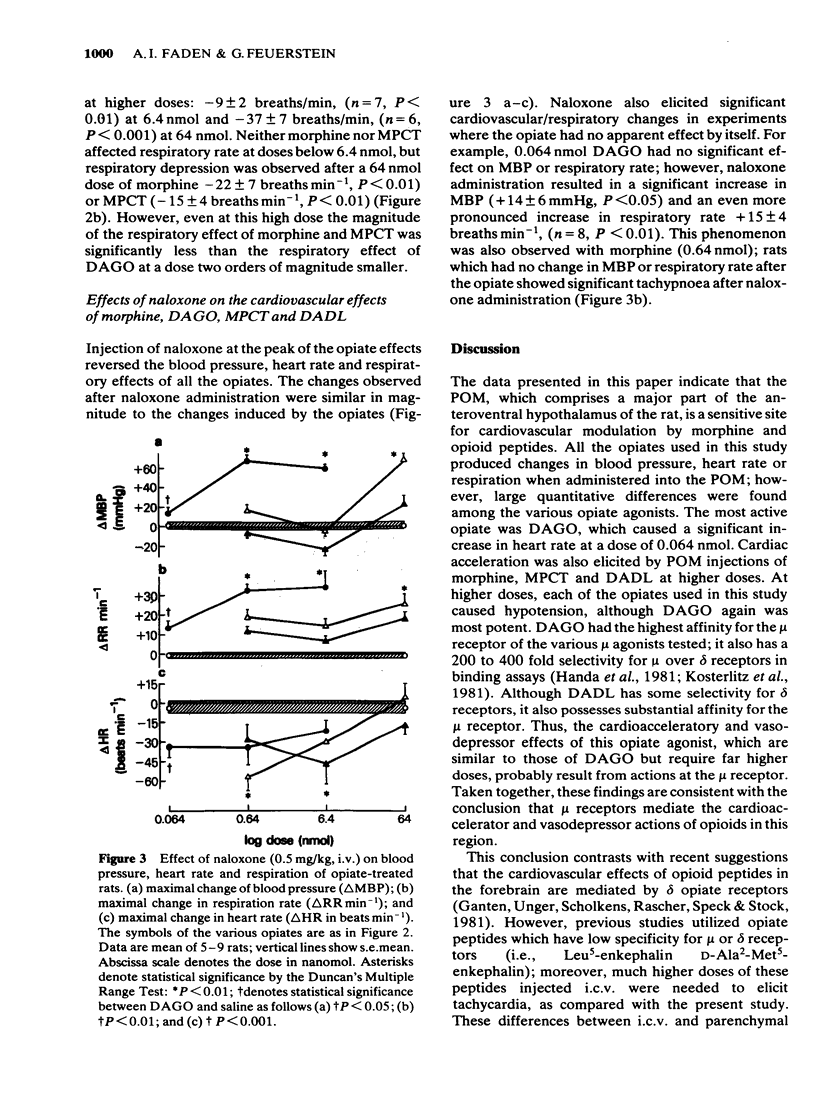
Experiments were designed to evaluate the role of mu and delta opiate receptors in central cardiovascular control in the hypothalamic nucleus preopticus medialis of rats anaesthetized with pentobarbitone. The highly selective mu opiate receptor agonist D-Ala2-MePhe4-Gly5-ol-enkephalin was extremely potent in eliciting hypotension and bradypnoea; tachycardia was elicited by a low dose (0.064 nmol), but not by higher doses (0.64-6.4 nmol). Other selective mu receptor agonists (morphine sulphate, morphiceptin) caused tachycardia at lower doses (0.64, 6.4 nmol), hypotension and bradypnoea after the highest dose (64 nmol). The relatively selective delta receptor agonist D-Ala2-D-Leu5-enkephalin caused profound bradypnoea and hypotension at the high dose (64 nmol), tachycardia after the lowest dose (0.64 nmol), but bradycardia was found during the hypotension induced by the high dose (64 nmol). All of the opiate/opioid effects were reversed by naloxone (0.5 mg kg-1, i.v.). It is concluded that mu receptors may mediate the cardiovascular and respiratory effects of opiates and opioid peptides in the nucleus preopticus medialis of the rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolme P., Fuxe K., Agnati L. F., Bradley R., Smythies J. Cardiovascular effects of morphine and opioid peptides following intracisternal administration in chloralose-anesthetized rats. Eur J Pharmacol. 1978 Apr 1;48(3):319–324. doi: 10.1016/0014-2999(78)90090-0. [DOI] [PubMed] [Google Scholar]
- Brody M. J., Haywood J. R., Touw K. B. Neural mechanisms in hypertension. Annu Rev Physiol. 1980;42:441–453. doi: 10.1146/annurev.ph.42.030180.002301. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Lillian A., Hazum E., Cuatrecasas P., Chang J. K. Morphiceptin (NH4-tyr-pro-phe-pro-COHN2): a potent and specific agonist for morphine (mu) receptors. Science. 1981 Apr 3;212(4490):75–77. doi: 10.1126/science.6259732. [DOI] [PubMed] [Google Scholar]
- Faden A. I., Holaday J. W. Experimental endotoxin shock: the pathophysiologic function of endorphins and treatment with opiate antagonists. J Infect Dis. 1980 Aug;142(2):229–238. doi: 10.1093/infdis/142.2.229. [DOI] [PubMed] [Google Scholar]
- Farber J. P., Connors A. F., Gisolfi C. V., McCaffree D. R., Smith R. M. Effects on breathing of putative neurotransmitters in the rostral hypothalamus of the rat. Brain Res Bull. 1981 Jan;6(1):13–17. doi: 10.1016/s0361-9230(81)80064-0. [DOI] [PubMed] [Google Scholar]
- Farsang C., Kunos G. Naloxone reverses the antihypertensive effect of clonidine. Br J Pharmacol. 1979 Oct;67(2):161–164. doi: 10.1111/j.1476-5381.1979.tb08661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein G., Faden A. I. Differential cardiovascular effects of mu, delta and kappa opiate agonists at discrete hypothalamic sites in the anesthetized rat. Life Sci. 1982 Nov 15;31(20-21):2197–2200. doi: 10.1016/0024-3205(82)90117-5. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa B. K., Land A. C., Lord J. A., Morgan B. A., Rance M. J., Smith C. F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol. 1981 Apr 9;70(4):531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Hassen A. H., Feuerstein G., Faden A. I. mu Receptors and opioid cardiovascular effects in the NTS of rat. Peptides. 1982 Nov-Dec;3(6):1031–1037. doi: 10.1016/0196-9781(82)90074-2. [DOI] [PubMed] [Google Scholar]
- Hassen A. H., Feuerstein G., Pfeiffer A., Faden A. I. Delta versus mu receptors: cardiovascular and respiratory effects of opiate agonists microinjected into nucleus tractus solitarius of cats. Regul Pept. 1982 Nov;4(6):299–309. doi: 10.1016/0167-0115(82)90140-9. [DOI] [PubMed] [Google Scholar]
- Holaday J. W., Faden A. I. Naloxone acts at central opiate receptors to reverse hypotension, hypothermia and hypoventilation in spinal shock. Brain Res. 1980 May 5;189(1):295–300. doi: 10.1016/0006-8993(80)90032-3. [DOI] [PubMed] [Google Scholar]
- Hurlé M. A., Mediavilla A., Flórez J. Morphine, pentobarbital and naloxone in the ventral medullary chemosensitive areas: differential respiratory and cardiovascular effects. J Pharmacol Exp Ther. 1982 Mar;220(3):642–647. [PubMed] [Google Scholar]
- Janssen H. F., Lutherer L. O. Ventriculocisternal administration of naloxone protects against severe hypotension during endotoxin shock. Brain Res. 1980 Aug 4;194(2):608–612. doi: 10.1016/0006-8993(80)91251-2. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lord J. A., Paterson S. J., Waterfield A. A. Effects of changes in the structure of enkephalins and of narcotic analgesic drugs on their interactions with mu- and delta-receptors. Br J Pharmacol. 1980 Feb;68(2):333–342. doi: 10.1111/j.1476-5381.1980.tb10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Paterson S. J., Robson L. E. Characterization of the kappa-subtype of the opiate receptor in the guinea-pig brain. Br J Pharmacol. 1981 Aug;73(4):939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubie M., Schmitt H., Vincent M., Remond G. Central cardiovascular effects of morphinomimetic peptides in dogs. Eur J Pharmacol. 1977 Nov 1;46(1):67–71. doi: 10.1016/0014-2999(77)90146-7. [DOI] [PubMed] [Google Scholar]
- Law P. Y., Loh H. H., Li C. H. Properties and localization of beta-endorphin receptor in rat brain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5455–5459. doi: 10.1073/pnas.76.11.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- McGilliard K. L., Takemori A. E. Antagonism by naloxone of narcotic-induced respiratory depression and analgesia. J Pharmacol Exp Ther. 1978 Nov;207(2):494–503. [PubMed] [Google Scholar]
- Petty M. A., Reid J. L. The effect of opiates on arterial baroreceptor reflex function in the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):206–211. doi: 10.1007/BF00495866. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Pasi A., Mehraein P., Herz A. Opiate receptor binding sites in human brain. Brain Res. 1982 Sep 23;248(1):87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- Simantov R., Kuhar M. J., Uhl G. R., Snyder S. H. Opioid peptide enkephalin: immunohistochemical mapping in rat central nervous system. Proc Natl Acad Sci U S A. 1977 May;74(5):2167–2171. doi: 10.1073/pnas.74.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsen J. M., Van Ree J. M., De Jong W. Cardiovascular and respiratory effects of beta-endorphin in anesthetized and conscious rats. J Cardiovasc Pharmacol. 1982 Nov-Dec;4(6):883–888. [PubMed] [Google Scholar]
- Yukimura T., Stock G., Stumpf H., Unger T., Ganten D. Effects of [D-Ala2]-methionine-enkephalin on blood pressure, heart rate, and baroreceptor reflex sensitivity in conscious cats. Hypertension. 1981 Sep-Oct;3(5):528–533. doi: 10.1161/01.hyp.3.5.528. [DOI] [PubMed] [Google Scholar]
- Zobrist R. H., Allerton H. W., Isom G. E. Characterization of the respiratory activity of (D-Ala2)methionine-enkephalinamide. Eur J Pharmacol. 1981 Mar 12;70(2):121–128. doi: 10.1016/0014-2999(81)90206-5. [DOI] [PubMed] [Google Scholar]


