Abstract
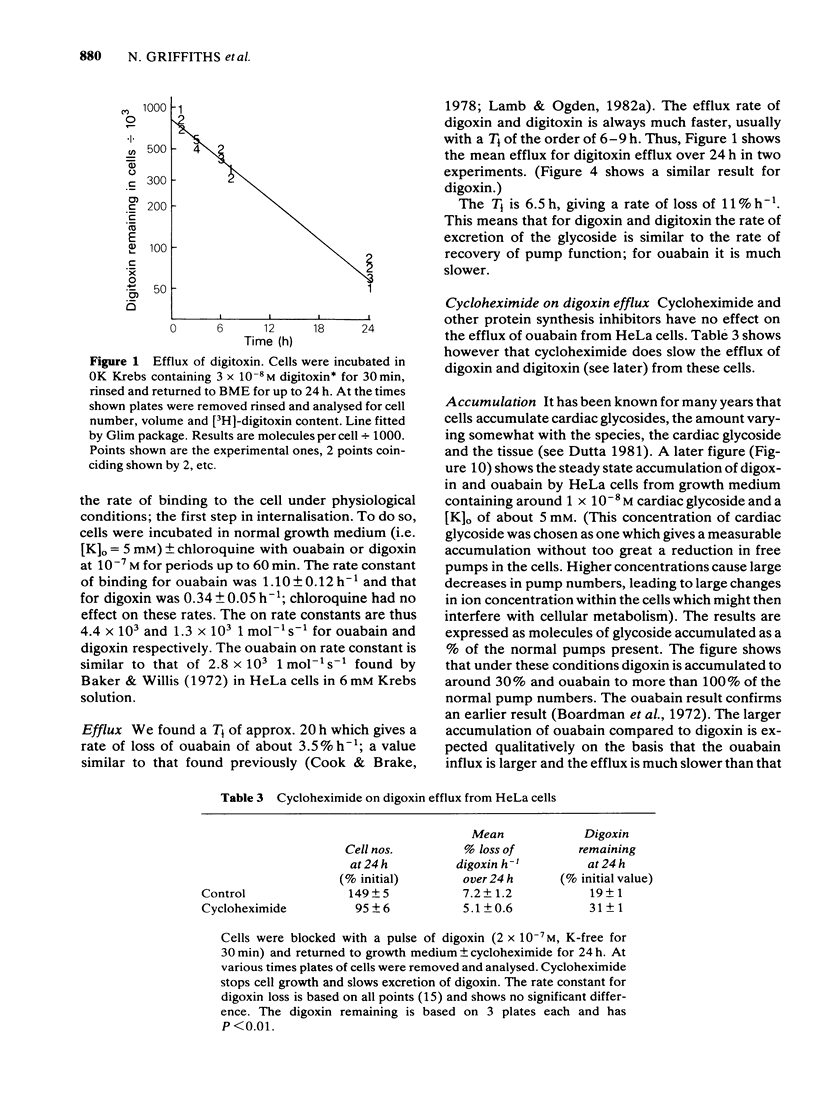
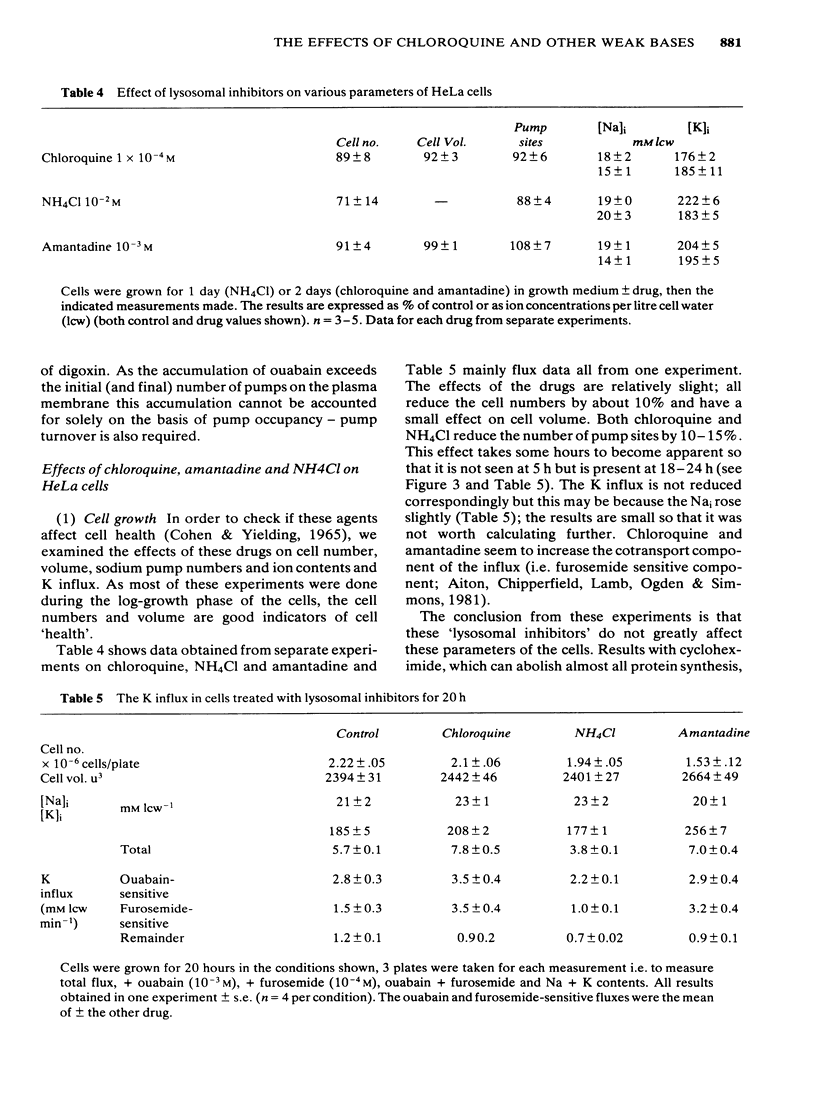
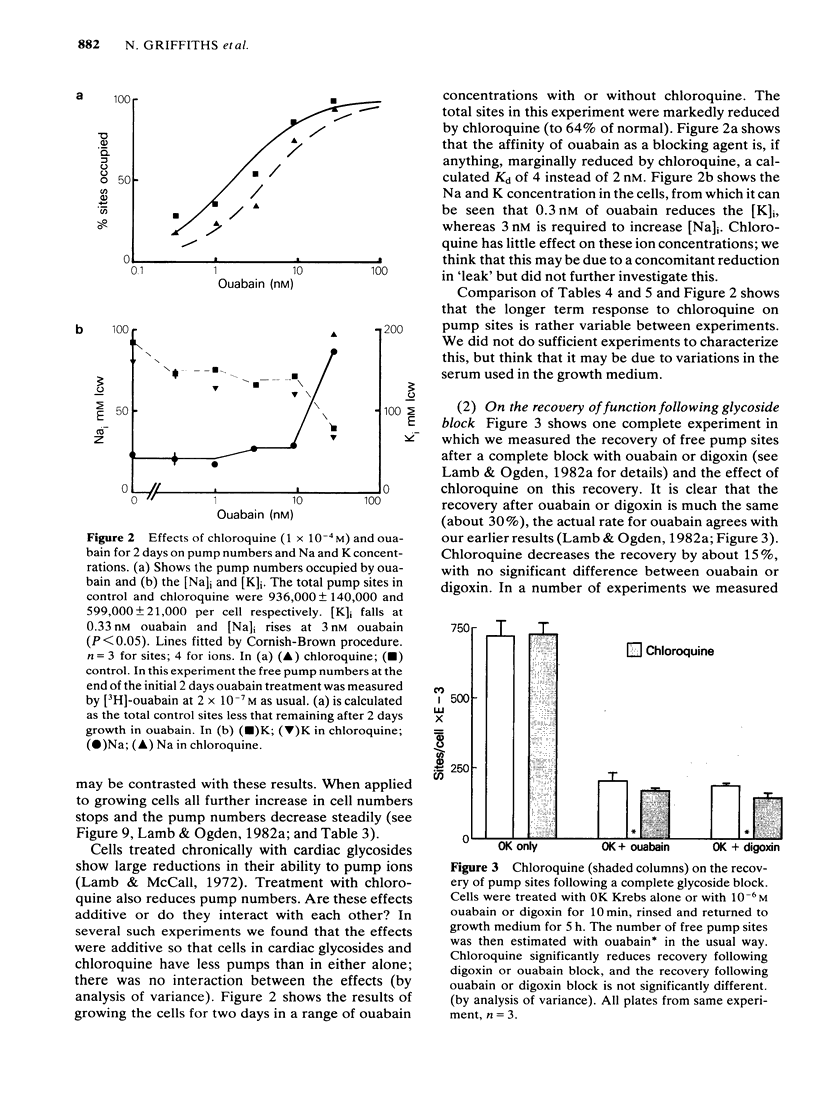
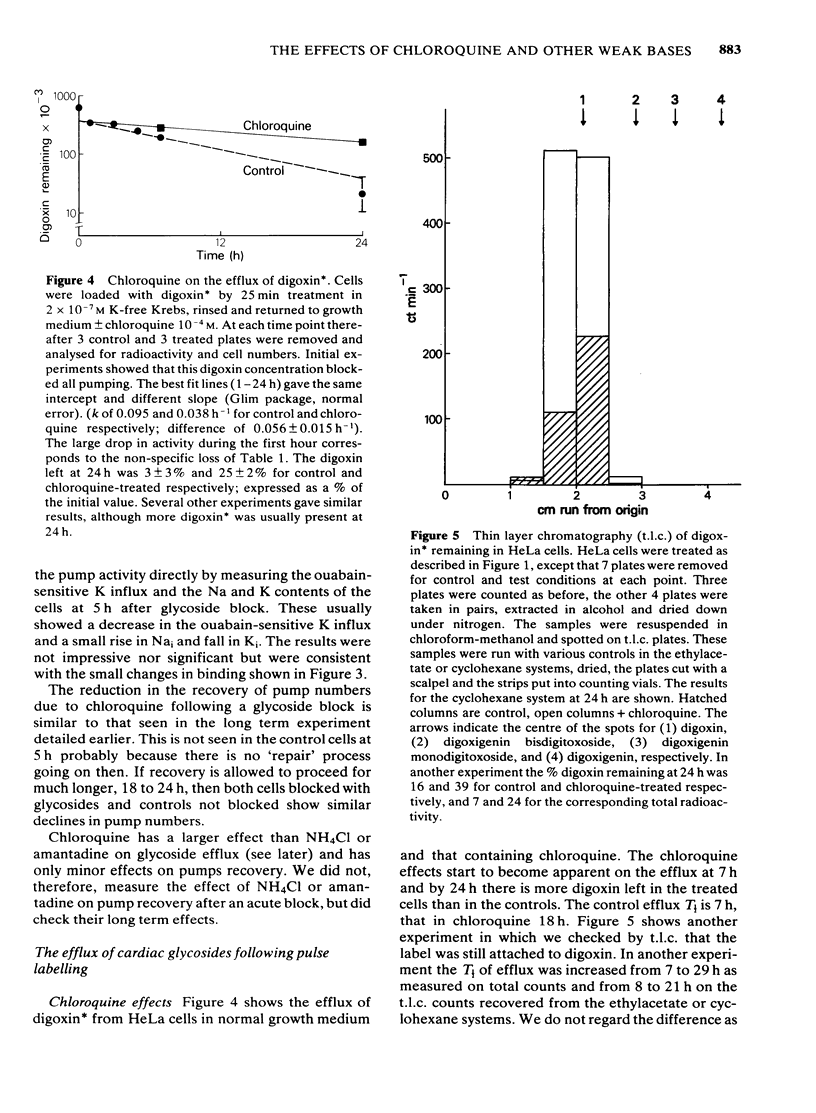
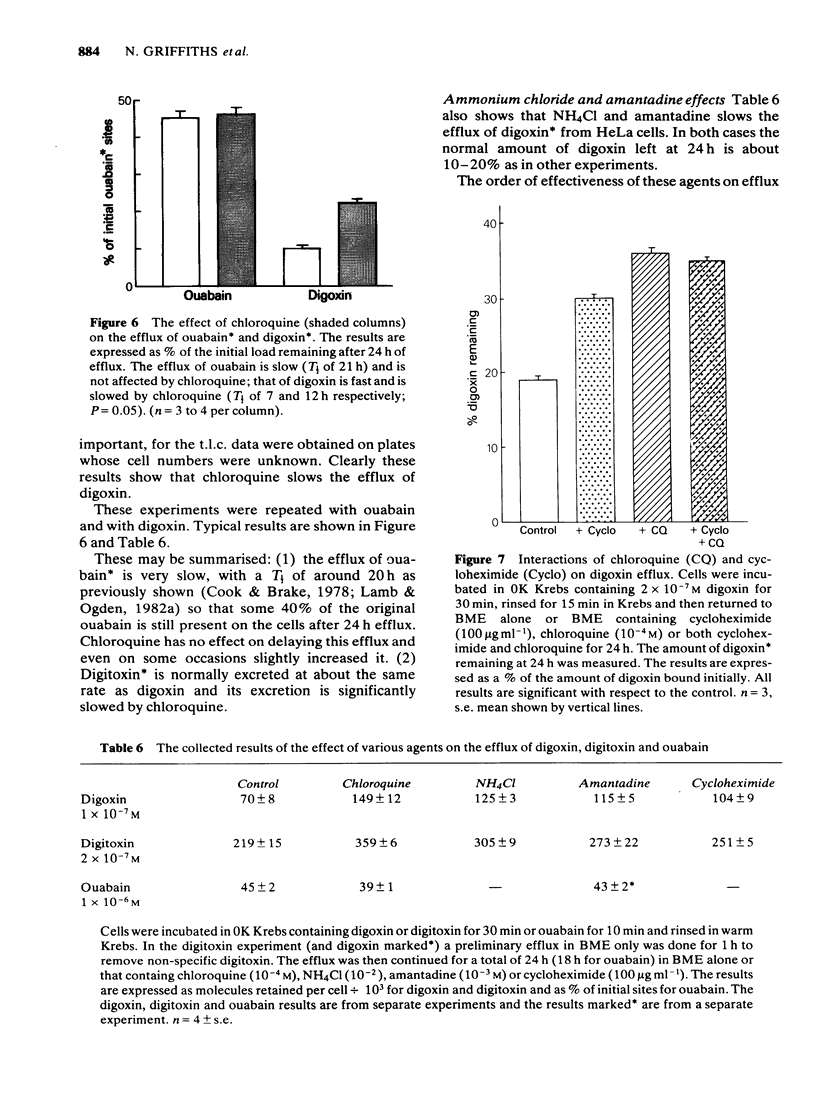
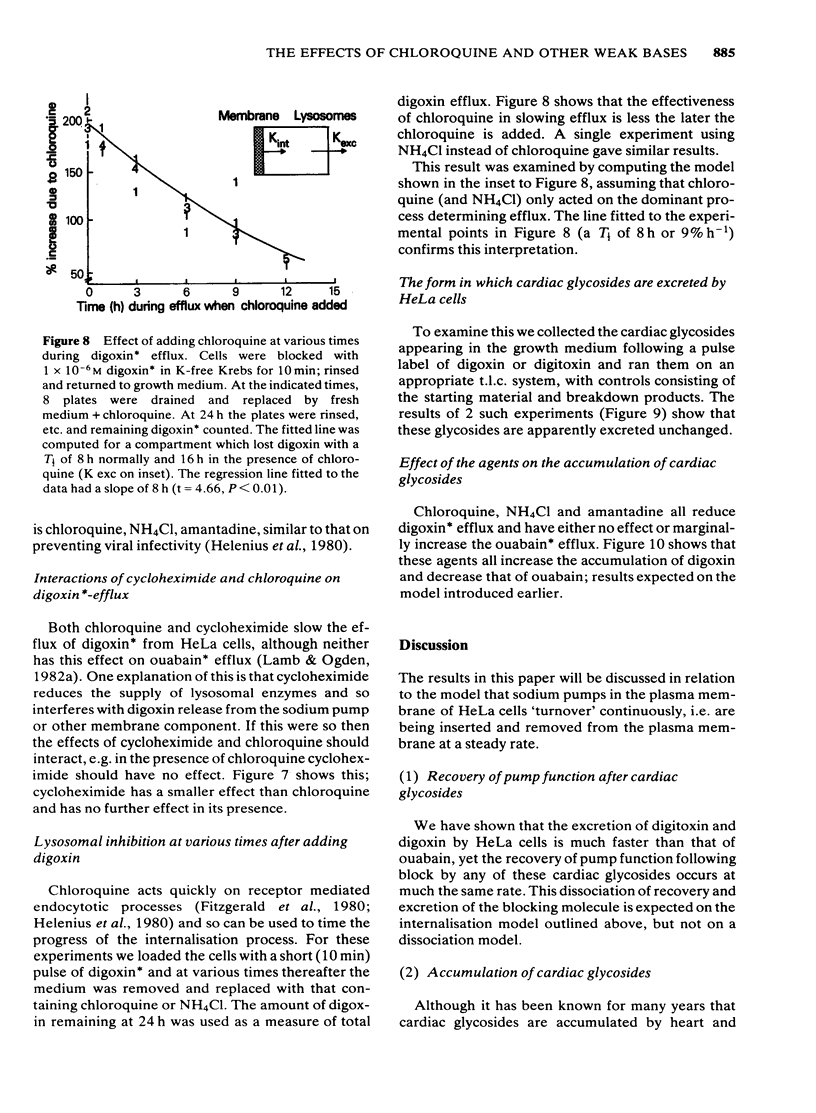
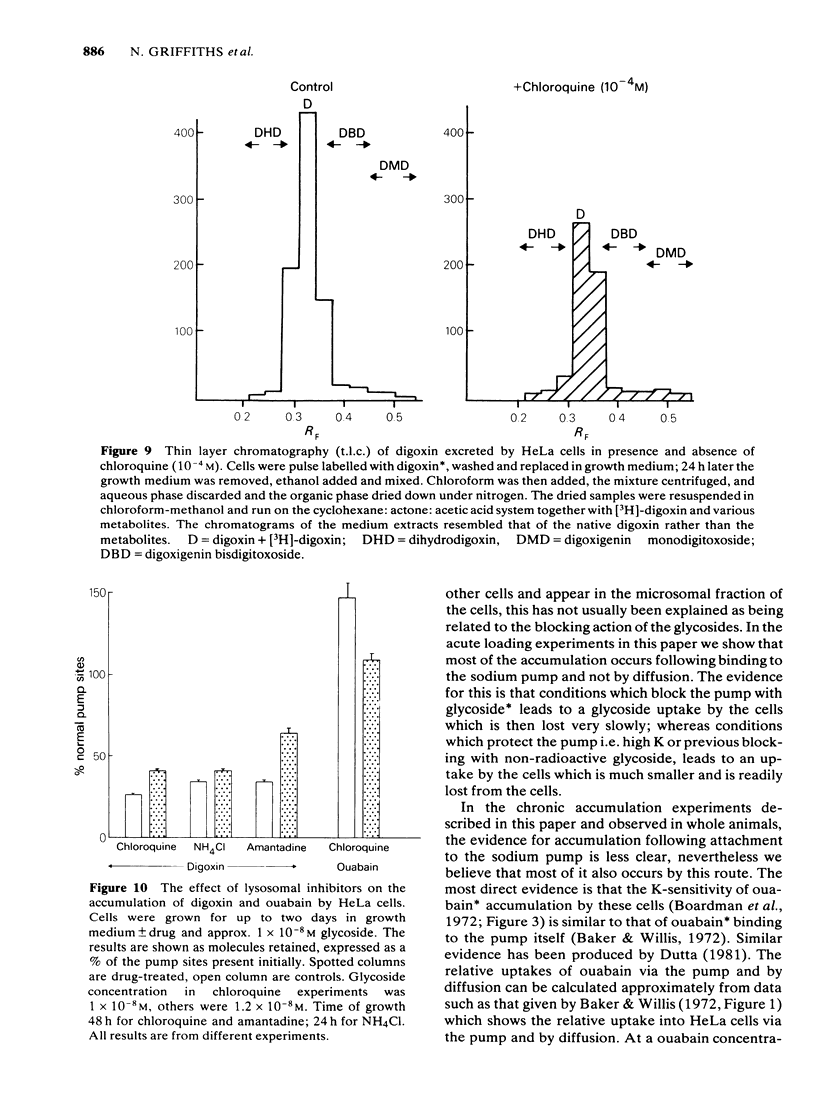
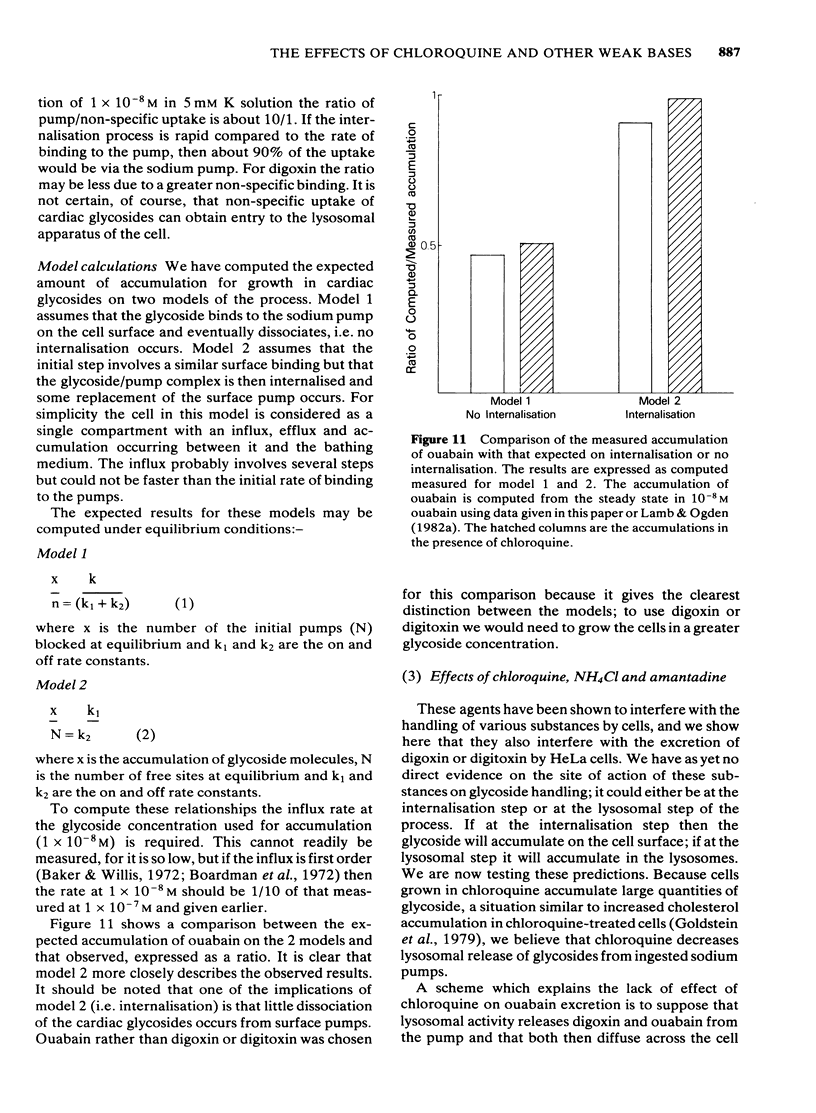
We have studied the effects of the weak bases chloroquine, NH4Cl and amantadine on the handling of certain cardiac glycosides by HeLa cells. When these weak bases are applied acutely to HeLa cells they have only minor effects on the binding of cardiac glycosides to the sodium pumps and on the recovery of pump function following block. When cells are grown in these weak bases there is a variable (10-30%) reduction in pump numbers. This effect is additive to that of chronic treatment with cardiac glycosides. If all sodium pumps are blocked with ouabain, digoxin or digitoxin then recovery of function recovers with a T1/2 of about 7 h (10% h-1); digoxin and digitoxin molecules are excreted at a similar rate but ouabain excretion occurs at a much slower rate (3% h-1). These weak bases greatly slow (x 3) the rate of excretion of digoxin and digitoxin but do not alter that of ouabain. The process affected by chloroquine was estimated to have a T1/2 of 8 h. Cells grown in the presence of cardiac glycosides accumulate large numbers of glycoside molecules; chloroquine, NH4Cl and amantadine increase the accumulation of digoxin and digitoxin and may decrease that of ouabain. Quantitatively these results fit a model whereby cardiac glycosides are accumulated by HeLa cells bound to the sodium pumps, are processed by the lysosomes and then excreted. The results are consistent with a process of internalisation and renewal of sodium pumps by HeLa cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Chipperfield A. R., Lamb J. F., Ogden P., Simmons N. L. Occurrence of passive furosemide-sensitive transmembrane potassium transport in cultured cells. Biochim Biophys Acta. 1981 Sep 7;646(3):389–398. doi: 10.1016/0005-2736(81)90307-2. [DOI] [PubMed] [Google Scholar]
- Aiton J. F., Lamb J. F., Ogden P. Down-regulation of the sodium pump following chronic exposure of HeLa cells and chick embryo heart cells to ouabain. Br J Pharmacol. 1981 Jun;73(2):333–340. doi: 10.1111/j.1476-5381.1981.tb10426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS R., LAMB J. F. Sodium and potassium fluxes in cells cultured from chick embryo heart muscle. J Physiol. 1962 Aug;162:510–531. doi: 10.1113/jphysiol.1962.sp006947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Binding of the cardiac glycoside ouabain to intact cells. J Physiol. 1972 Jul;224(2):441–462. doi: 10.1113/jphysiol.1972.sp009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L. J., Lamb J. F., McCall D. Uptake of ( 3 H)ouabain and Na pump turnover rates in cells cultured in ouabain. J Physiol. 1972 Sep;225(3):619–635. doi: 10.1113/jphysiol.1972.sp009960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L., Huett M., Lamb J. F., Newton J. P., Polson J. M. Evidence for the genetic control of the sodium pump density in HeLa cells. J Physiol. 1974 Sep;241(3):771–794. doi: 10.1113/jphysiol.1974.sp010684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhas M. L., Figueira M. A. Comparative study of thin-layer chromatographic techniques for separation of digoxin, digitoxin and their main metabolites. J Chromatogr. 1973 Nov 7;86(1):254–260. doi: 10.1016/s0021-9673(01)81267-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Yielding K. L. Inhibition of DNA and RNA polymerase reactions by chloroquine. Proc Natl Acad Sci U S A. 1965 Aug;54(2):521–527. doi: 10.1073/pnas.54.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D., Morris R. E., Saelinger C. B. Receptor-mediated internalization of Pseudomonas toxin by mouse fibroblasts. Cell. 1980 Oct;21(3):867–873. doi: 10.1016/0092-8674(80)90450-x. [DOI] [PubMed] [Google Scholar]
- Fletcher G. L., Stainer I. M., Holmes W. N. Sequential changes in the adenosinetriphosphatase activity and the electrolyte excretory capacity of the nasal glands of the duck (Anas platyrhynchos) during the period of adaptation to hypertonic saline. J Exp Biol. 1967 Dec;47(3):375–391. doi: 10.1242/jeb.47.3.375a. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Kilno D. R., Swartz T. J., Butler V. P., Jr Effects of digoxin-specific antibodies on accumulation and binding of digoxin by human erythrocytes. J Clin Invest. 1973 Aug;52(8):1820–1833. doi: 10.1172/JCI107364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gotlib L. J. Isolation of cell plasma membranes on microcarrier culture beads. Biochim Biophys Acta. 1982 Feb 8;685(1):21–26. doi: 10.1016/0005-2736(82)90029-3. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., Boardman L. J., Newton J. P., Aiton J. F. Effect of calf serum on sodium pump density in HeLa cells. Nat New Biol. 1973 Mar 28;242(117):115–117. doi: 10.1038/newbio242115a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., McCall D. Effect of prolonged ouabain treatment of Na, K, Cl and Ca concentration and fluxes in cultured human cells. J Physiol. 1972 Sep;225(3):599–617. doi: 10.1113/jphysiol.1972.sp009959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., Ogden P. Internalization of ouabain and replacement of sodium pumps in the plasma membranes of HeLa cells following block with cardiac glycosides. Q J Exp Physiol. 1982 Jan;67(1):105–119. doi: 10.1113/expphysiol.1982.sp002605. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., Ogden P. The turnover of sodium pumps in HeLa cells [proceedings]. J Physiol. 1977 Jul;269(1):79P–80P. [PubMed] [Google Scholar]
- Lo C. S., Edelman I. S. Effect of triiodothyronine on the synthesis and degradation of renal cortical (Na+ + k+)-adenosine triphosphatase. J Biol Chem. 1976 Dec 25;251(24):7834–7840. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack L. R., Tate E. H., Cook J. S. Turnover and regulation of Na-K-ATPase in HeLa cells. Am J Physiol. 1981 Nov;241(5):C173–C183. doi: 10.1152/ajpcell.1981.241.5.C173. [DOI] [PubMed] [Google Scholar]
- SJOERDSMA A., FISCHER C. S. The fixation of radioactive digitoxin by isolated hearts. Circulation. 1951 Jul;4(1):100–104. doi: 10.1161/01.cir.4.1.100. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Titus-Dillon P., Hall C. W., Neufeld E. F. Inhibition of receptor-mediated uptake of a lysosomal enzyme into fibroblasts by chloroquine, procaine and ammonia. Exp Cell Res. 1979 Mar 15;119(2):359–364. doi: 10.1016/0014-4827(79)90364-1. [DOI] [PubMed] [Google Scholar]


