Abstract
Type IV secretory systems are a group of bacterial transporters responsible for the transport of proteins and nucleic acids directly into recipient cells. Such systems play key roles in the virulence of some pathogenic organisms and in conjugation-mediated horizontal gene transfer. Many type IV systems require conserved “coupling proteins,” transmembrane polypeptides that are critical for transporting secreted substrates across the cytoplasmic membrane of the bacterium. In vitro evidence suggests that the functional form of coupling proteins is a homohexameric, ring-shaped complex. Using a library of tagged mutants, we investigated the structural and functional organization of the F plasmid conjugative coupling protein TraD by coimmunoprecipitation, cross-linking, and genetic means. We present direct evidence that coupling proteins form stable oligomeric complexes in the membranes of bacteria and that the formation of some of these complexes requires other F-encoded functions. Our data also show that different regions of TraD play distinct roles in the oligomerization process. We postulate a model for in vivo oligomerization and discuss the probable participation of individual domains of TraD in each step.
Gram-negative bacteria use a variety of systems to export substrates across their inner and outer membranes. Among the most versatile of these translocation machines are the type IV secretion systems (T4SSs), which specialize in intercellular transport. Pathogenic organisms such as Legionella pneumophila and Bordetella pertussis use T4SSs to export effector proteins into host cells, and some T4SSs can secrete multiple substrates (8, 9). Conjugative plasmids often encode T4SSs that drive the transfer of plasmid DNA from donor cells to recipients (13, 30). The plant pathogen Agrobacterium tumefaciens secretes both proteins and DNA into plant cells to stably transform the plant with bacterial DNA, making its T4SS the only known transport system dedicated to interdomain genetic transfer (8).
T4SSs of gram-negative bacteria are structurally complex. They energize coupled translocation across both membranes using a large number of individual proteins that span the cytoplasmic membrane (CM), periplasmic space, and outer membrane (8, 9). All conjugative T4SSs and many virulence-associated T4SSs require a CM-spanning protein called the “coupling protein” (CP). CPs interact with cytoplasmic and extracytoplasmic components of the secretory system to couple substrate processing to substrate secretion and are therefore thought to form a gated pore for the T4SS, controlling the specificity of secretion by ferrying specific substrate molecules across the CM for delivery into the target cell (9, 15, 21, 34, 45, 46). In the prevailing model of CP function, CPs form ring-shaped homohexamers with central channels capable of accommodating secreted substrates. This model is based on the crystal structure of TrwBΔN70, a C-terminal portion of the TrwB CP from plasmid R388 (16, 17), as well as microscopic analysis of purified full-length TrwB (22) and in vitro evidence that suggests that purified CPs act as oligomers (16, 46, 47).
The ring-shaped structure of TrwBΔN70 bears remarkable similarity to model molecular motors such as the F1Fo ATPase, the Escherichia coli cell division protein FtsK, and the Bacillus subtilis sporulation protein SpoIIIE (17). CPs contain consensus ATP-binding domains (32), and a C-terminal domain of TrwB has been shown to have ATPase activity in vitro (49). These observations suggest that CPs act as molecular motors, harnessing the energy of ATP hydrolysis to effect structural changes and, thereby, molecular transport.
Because of the central role that CPs play in type IV secretion, a mechanistic picture of CP assembly and function will be critical for drawing a detailed model of protein and DNA transport through T4SSs. An understanding of CP structure could also shed light on the physical mechanisms used by molecular motors to couple ATP hydrolysis to large-scale motion. Using the TraD protein from the F plasmid conjugation system as our model, we demonstrate in vivo CP oligomerization by using coimmunoprecipitation (coIP) and protein cross-linking techniques. Analysis of various mutants suggests that TraD spontaneously forms back-to-front homodimers in the CM and that these dimers are assembled into a larger structure in a process facilitated by other F plasmid proteins. The association between monomers depends on sequences in the N-terminal 151 residues of the protein and includes interactions between the large C-terminal cytoplasmic domains of individual subunits. Our data are consistent with the prevailing model of coupling proteins as symmetric, ring-shaped homooligomers in the CM that interact with other secretory components in the cell envelope.
MATERIALS AND METHODS
Growth media, strains, and plasmids.
E. coli strains and plasmids used in this study are summarized in Table 1. The rich (LB) and minimal (M63) media (40) contained supplements at the following concentrations: 100 μg/ml ampicillin, 30 μg/ml chloramphenicol, 15 μg/ml tetracycline, 40 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and 1 mM isopropyl-β-d-galactopyranoside (IPTG).
TABLE 1.
Strains and plasmids used in this study
| E. coli strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| BT6 | MC1000 ΔmalB101 zjb::Tn5 (ΔmalEFGK lamB malM) | 27 |
| BT8 | MC4100 malTc | 50 |
| CC1254 | MC1000 ΔrecBCD::Red-Kan leu(s) phoAΔ20 rpoB argE (Am) | C. Manoil |
| HS3169 | MC4100 malKΔ16 zjb-729::Tn10 | 41 |
| XK1502 | F− ΔlacU169 nalA | 42 |
| Plasmids | ||
| F′42 | F′lac JCFL0 | 1 |
| F′ΔD | F′42 with traD deleted and replaced by Tn10 tetRA sequence | This study |
| pBT200 | pTrc99A with wild-type traD cloned into the MCS | 20 |
| pEG100 | pACYC184-derived; MCS from pTrc99A; trc promoter; lacIq | 20 |
| pEG103 | pEG100 with traD cloned into the MCS | 20 |
| pNLK5 | pBAD18 with traD cloned into the MCS; araBAD promoter | 31 |
| pTrc99A | Apr cloning vector; trc promoter; lacIq | 2 |
MCS, multiple cloning site.
Mutant traD alleles were subcloned from pNLK5-derived plasmids (31) into the pTrc99A vector by digesting the pNLK5 derivatives with EcoRI and XbaI and ligating the traD-containing fragment into the EcoRI and XbaI sites of the multiple cloning site of pTrc99A. The pTrc99A-derived plasmid containing wild-type traD was designated pBT200. traD alleles were subcloned from pTrc99A derivatives into the lower-copy-number pEG100 vector by ligation of traD-containing fragments after digestion with Acc65I and HindIII.
A derivative of the F plasmid lacking traD was generated as follows. The traD gene in F′42 (Table 1) was replaced with a PCR-amplified tetracycline resistance cassette from Tn10 by λ Red recombination (10) in strain CC1254 to generate F′42ΔtraD::tetRA (“F′ΔD”). The following primers were used for tetRA PCR amplification (homology to tetRA is indicated by uppercase type): cttcggaatatcatcatgagttttaacgcaaaggatatgTTAAGACCCACTTTCACATT (forward) and gagttgacctgttcatcagaaatcatctcccggcgcaaCTAAGCACTTGTCTCCTG (reverse). Only the first 23 coding nucleotides and the final 21 coding nucleotides of traD remained intact.
Construction of mutants.
The previously described i31 mutagenesis of traD on plasmid pNLK5 (31) was continued essentially as described previously. Approximately 4,000 colonies from the initial TnlacZ/in-transposition reaction were screened, resulting in 13 in-frame insertions. Of these insertions, 10 represented new traD::i31 alleles (with insertions after codons Q94, G282, and N702 represented in both mutant sets). A fusion between the N-terminal coding region of the RP4 TraG protein and the C-terminal coding region of TraD was generated using the unique BamHI site present in the i31 sequence of the traG::A119 (46) and traD::Q151 mutants, as previously described (39). In a similar fashion, an i31-tagged deletion lacking residues 7 to 134 was generated using the K6 and I133 insertion mutant plasmids. To generate an untagged mutant lacking residues 2 to 134, the coding sequence of traD was amplified from pBT200 by PCR using the following primers: GGATATATGAACCATGGGGCGTCAGGGTAAACAACAGAG (forward) and GGCTGAAAATCTTCTCTCATCCGCC (reverse).
The forward primer was designed to add an NcoI site to the PCR product upstream of the traD coding sequence. After PCR, the product was cut with NcoI and HindIII and ligated into the large NcoI/HindIII fragment of pTrc99A. The production of all mutant proteins was verified by Western blotting using polyclonal i31-specific (38) or TraD-specific (43) antiserum.
Radiolabeling, coIP, and detection of proteins.
coIP experiments were performed in BT8-derived strains carrying F′ΔD; wild-type TraD was expressed from pBT200 or pEG103, and mutants were expressed from pEG100-derived or pNLK5-derived plasmids (Table 1). Radiolabeling, precipitation, and detection of proteins, including pulse-chase experiments, were performed essentially as described previously (26). Important differences were as follows: immune binding and precipitations were performed in KK buffer (50 mM Tris Cl [pH 8.0], 1 mM EDTA, 150 mM NaCl, 0.01% dodecyl maltoside), proteins were incubated with a polyclonal i31 antiserum or a polyclonal anti-TraD antibody, and immune complexes were precipitated using IgGsorb (Enzyme Center). For pulse-chase experiments, a 5-min pulse was followed by a 15- or 20-min chase as noted.
Protein localization.
The localizations of several TraD derivatives were assessed in BT8-derived strains carrying F′ΔD and expressing traD alleles from pEG100-derived plasmids. Cultures were grown to an optical density at 600 nm of ∼0.3, and traD expression was induced with IPTG for 10 min to mimic conditions for coIPs. Cells were then sonicated with a probe sonicator for a total of 35 s at a 0.05-W output. Whole cells and any protein aggregates were removed from the lysate by two 10-min spins at 3,000 × g. Membranes were pelleted by centrifugation at 128,000 × g for 1 h. Proteins were resolved on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and then transferred onto nitrocellulose overnight at 4°C at 300 mA in a buffer containing 20% methanol, 3 mM Na2CO3, and 10 mM NaHCO3. Proteins of interest were detected by Western blotting with i31-specific, MalE-specific (New England Biolabs), LacZ-specific (5 Prime→3 Prime Inc.), or MalF-specific (50) antiserum. A BT6 cell extract was used as the negative control for all Western blots.
Protein cross-linking.
Cross-linking experiments were performed on XK1502 derivatives expressing traD alleles from pTrc99A-derived vectors. Cultures were grown in rich medium to an optical density at 600 nm of ∼0.3. Cells were washed twice in phosphate-buffered saline and then resuspended in one-quarter the original volume in phosphate-buffered saline. An aliquot was taken to serve as an un-cross-linked control. Dithiobis(succinimidylpropionate) (DSP; Pierce) was added to the remainder from a freshly made stock of 10 mM in dimethyl sulfoxide, and the suspensions were incubated for 30 min at room temperature. The cross-linking reaction was quenched with Tris Cl (pH 7.4) (final concentration of 200 mM) and incubated for 15 min at room temperature. To generate a “cross-linked, cleaved” sample, half of the cross-linked sample was transferred into a fresh tube with dithiothreitol (Sigma) (final concentration of 50 mM). Samples were pelleted in a microcentrifuge for 10 min at 16,000 × g. The pellets were resuspended in loading buffer with (for un-cross-linked and cross-linked, cleaved) or without (for cross-linked, uncleaved) β-mercaptoethanol. Proteins were resolved on an 8% SDS-polyacrylamide gel and transferred onto nitrocellulose. TraD and TraDi31 mutants were detected by Western blotting with a polyclonal TraD-specific or i31-specific antiserum.
Conjugative complementation and dominance experiments.
XK1502-derived donors carrying F′ΔD (for complementation) or F′42 (for dominance) were used for conjugation studies. Equal portions (0.1 ml) of donor and HS3169 recipient cultures grown overnight (0.1 ml) were added to 0.8 ml LB, and the mixture was rotated slowly at 37°C for 45 min. Transconjugants were selected on lactose-minimal agar supplemented with leucine, isoleucine, and streptomycin. The promoter expressing traD in the donors was not induced during conjugation.
Computational modeling of TraD structure.
The C-terminal domain sequence from TraD was modeled on the previously reported structure of TrwBΔN70 (Protein Data Bank [PDB] accession number 1GL6) (17). The model was built from an alignment of TraD residues 135 to 576 to chain A of the structure reported under PDB accession number 1GL6 using the K*Sync program (6) to obtain coordinates for conserved positions, followed by the modeling of variable regions with loop modeling (44) and side-chain repacking (28) using Rosetta (48) via the Robetta server (7) (http://robetta.org/). These single-chain models were then fit to the six chains of the structure reported under PDB accession number 1GL6 to make a hexameric model.
RESULTS
λTnlacZ/in mutagenesis was used to generate insertion-tagged TraD mutants.
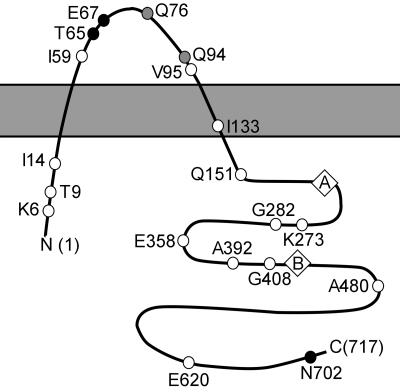
Previously, we isolated a preliminary set of mutations in the F plasmid traD gene with the λTnlacZ/in system and determined the transmembrane topology of TraD (31). The original mutant set has relatively few isolates with lesions in the large C-terminal cytoplasmic domain. To address this lack of coverage, we isolated 10 more mutations (Fig. 1), again by λTnlacZ/in mutagenesis, which encode full-length proteins with a defined 31-residue insertion (the “i31”) at the site of initial transposon insertion. The i31 disrupts nearby protein folding, which can be neutral or deleterious to function depending upon its location. In addition to informing topology studies for transmembrane proteins, i31 libraries have been used to study protein-protein interactions and map functional domains (26, 31, 33, 46, 51, 52). Mutant alleles and proteins are referred to by the last intact codon prior to the insertion site (e.g., TraDiN702 or N702).
FIG. 1.
Map of TraD showing membrane topology and positions of i31 mutations. The solid gray bar represents the cytoplasmic membrane; i31 insertion sites are shown along the length of TraD as circles. Diamonds represent consensus Walker A and B ATP-binding motifs. The color of the circle corresponds to the phenotype of the mutant in conjugative complementation assays. White, nonfunctional (<1% of control); gray, partially functional (1 to 50% of control); black, functional (>50% of control). Mutants generated for this study are T9, Q76, V95, I133, Q151, K273, E358, A392, G408, and A480.
To determine the phenotypes of the newly generated mutants in conjugation, donor bacteria that carried an F′lac plasmid with a nonpolar traD deletion were transformed with cloning vectors carrying traD::i31 alleles and assayed for their ability to donate F′ to recipients (Table 2). All of the newly generated mutants were very defective in conjugative complementation assays, with the exception of TraDiQ76, which complemented to 50% of wild-type conjugation frequency. All mutant proteins were stably expressed in donors except for TraDiV95. A summary of the entire TraD mutant library, showing locations of i31 inserts and the conjugation phenotypes of the mutants, is given in Fig. 1.
TABLE 2.
Conjugative phenotypes of TraD mutants
| TraD-expressing vector | Complementationa transconjugants per donor (SD) | Dominance,b % of WT conjugation |
|---|---|---|
| pNLK5 | 0.30 (0.05) | NT |
| pBT200 | 0.8 (0.1) | 100 |
| pBT200DiK6 | 4.5 × 10−7 (3.8 × 10−7) | 80 |
| pNLK5DiT9 | <3.1 × 10−8 | NT |
| pNLK5DiQ76 | 0.15 (0.01) | NT |
| pNLK5DiV95 | <2.3 × 10−8 | NT |
| pNLK5DiI133 | <3.5 × 10−7 | NT |
| pNLK5DiQ151 | 2 × 10−6 (2 × 10−6) | NT |
| pBT200DiK273 | <8 × 10−7 | 1.3 |
| pBT200DiG282 | <1.2 × 10−8 | 4.6 |
| pBT200DiE358 | 0.0010 (0.0003) | 100 |
| pBT200DiA392 | <5.5 × 10−7 | 15 |
| pBT200DiG408 | 1.4 × 10−6 (1 × 10−7) | 115 |
| pNLK5DiA480 | <3.1 × 10−8 | NT |
| pBT200DiN702 | 0.9 (0.1) | NT |
| pBT200DΔ2-134 | <2.8 × 10−8 | 139 |
| pEG100G-D | <1.9 × 10−7 | 146 |
Number given is the conjugation transfer frequency expressed as transconjugants/donor in a standard mating. Where detectable complementation was observed, frequencies were calculated with data from at least two independent replicates. Frequencies were measured in the XK1502 F′ΔtraD background. Uncomplemented XK1502 F′ΔtraD never gave rise to transconjugants. Values prefaced with the a “less-than” sign indicate alleles that did not detectably complement XK1502 F′ΔtraD for conjugation. Standard deviations are indicated parenthetically.
Dominance of traD alleles is given as the percentage of F′42 transfer from an XK1502 F′42 donor carrying the given plasmid compared to an XK1502 F′42-derived donor expressing wild-type (WT) traD from the same replicon. The mean frequency of transfer for XK1502 F′42 pBT200 was 0.54 transconjugants/donor. NT, not tested.
The cytoplasmic domain of TraD was structurally modeled based on homology to TrwBΔN70.
Homology modeling for residues 135 to 576 of the F plasmid TraD protein was performed using the structure of TrwBΔN70, the C-terminal cytoplasmic domain of the R388 (IncW) coupling protein TrwB (17), as a guide. Representative views of the TraD model are shown in Fig. 2. Most of the variability between TraD and TrwB (in particular the loop regions) was not at the interfaces between chains. The model is therefore likely to be accurate at the interfaces between monomers and is useful in interpreting the effects of lesions in these regions.
FIG. 2.
Ribbon diagram model of the C-terminal structure of a TraD hexamer. The model encompasses residues 135 to 576 of TraD. In each view, one monomer is darkened and thickened for clarity. Images were generated using the Visual Molecular Dynamics software package (25). (A) End view from cytoplasmic side along pore. Residues K273, E358, and A392 are shown in black and labeled. A and B faces are indicated by chevrons. (B) Side view, with membrane-proximal residues at the bottom.
Our homohexameric model of the C-terminal cytoplasmic domain of TraD suggested extensive contact between the cytoplasmic domains of constituent monomers. Three mutants in the library (K273, E358, and A392) were predicted to have lesions near subunit interfaces. We focused on these mutants as being potentially informative about the process of TraD oligomerization.
coIP demonstrates in vivo formation of stable TraD oligomers.
To assess the association between TraD derivatives in vivo, we coexpressed i31-tagged and untagged alleles, radiolabeled cells with [35S]methionine, and immunoprecipitated proteins with an i31-specific antibody. Whole-cell extracts and immunoprecipitates were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE); bands representing tagged and untagged TraD were quantified by phosphorimager analysis. The ratio of tagged to untagged protein in immunoprecipitated material was used as a measure of oligomerization. Summarized coIP results and representative gel lanes are shown in Fig. 3. Note that the method used here does not determine the oligomeric state of the complex but shows merely whether or not tagged and untagged proteins were interacting in the cell envelope.
FIG. 3.
coIP of TraD species. (A) Each bar represents coIP results for the pair of traD alleles listed above the bar. The height of the bar indicates the amount of untagged protein in the immunoprecipitated material relative to a wild-type (WT)/N702 positive control. Most i31-tagged traD alleles were expressed from pEG100-derived plasmids and paired with wild-type traD expressed from pBT200. The mean ratio of TraD to TraDiN702 in positive controls with these vectors was 0.47:0.53. traD::i31 alleles K6, I59, Q94, and G408 were expressed from pNLK5-derived plasmids and paired with traD expressed from pEG103. The ratio of TraD to TraDiN702 in the positive control with these vectors was 0.26:0.74. Error bars represent standard errors of the means for experiments performed at least twice. Mutants with i31 insertions in the C-terminal cytoplasmic domain are shown in white, those with N-terminal i31 insertions are shown in gray, and those with deleted or nonnative N-terminal domains are shown in black. (B) Sample gel lanes depicting whole-cell extracts (lanes 1, 3, 5, and 7) and immunoprecipitated proteins (lanes 2, 4, 6, and 8). Coexpressed species are noted above each pair of lanes. Mobilities of wild-type TraD, TraD::i31 mutants, TraDiΔ7-134, and G-D are shown at either side. (C) Western blot demonstrating localization of G-D, TraDiN702, and TraDiΔ7-134. Lane numbers, sample types, and TraD species are noted above the blot; protein mobilities are shown at the left. Marker bands are labeled with their sizes in kDa. The asterisk on the right indicates the mobility of soluble breakdown products of G-D and TraDiN702. All lanes contained material from similar numbers of cells. 702, TraDiN702; iΔ, TraDiΔ7-134.
TraDiN702 was used as a positive control in these experiments, as this mutant was highly functional in conjugation assays (31). When expressed in the presence of excess TraD, TraDiN702 precipitated nearly equivalent amounts of wild-type protein, demonstrating an in vivo association between the two. The C-terminal i31 mutants K273, E358, A392, and G408 precipitated wild-type TraD at levels approximating that of the N702 control, as did the N-terminal mutants K6, I59, and Q94. In contrast, the i31-tagged TraDiΔ7-134 mutant, truncated from the N terminus through the transmembrane domains, did not efficiently precipitate wild-type TraD. In the converse experiment, the untagged TraDΔ2-134 deletion failed to interact with TraDiN702. Both deletion mutants are expressed at levels comparable to those of wild-type TraD as determined by Western blotting and are present at similar levels in radiolabeled whole-cell extracts (Fig. 3B and data not shown). DiΔ7-134 failed to interact with DΔ2-134 by coIP, demonstrating that the cytoplasmic C-terminal domains of TraD do not interact in vivo in the absence of the transmembrane domains. These data indicate a requirement for the N-terminal domain in the association between monomers.
A requirement for the N-terminal domain of TraD could be the result of a sequence-specific interaction between monomers or could indicate the importance of membrane insertion per se. To distinguish between these possibilities, we constructed the G-D fusion protein, which consists of the first 119 residues (including the transmembrane domains) from the homologous RP4 CP TraG fused to the C-terminal cytoplasmic domain of TraD. Strikingly, G-D failed to interact with wild-type TraD in coIP experiments; therefore, we conclude that there are specific sequences in the first 151 residues of the protein required for TraD monomers to bind one another. The critical sequences likely lie in the transmembrane domains, as TraDiK6 and TraDiI59, with insertions in the N-terminal cytoplasmic and periplasmic domains, respectively, efficiently precipitated wild-type TraD in this assay.
The above-mentioned interpretation assumes that G-D localizes appropriately to the cell envelope. To ensure that the G-D protein was indeed membrane inserted, we separated cell lysates into a soluble fraction (cytoplasm and periplasm) and a membrane fraction (outer membrane and cytoplasmic membrane). We then compared the localizations of the G-D fusion to TraDiN702, TraDiΔ7-134, MalE, MalF, and LacZ. The full-length G-D fusion exhibited the same fractionation pattern as TraDiN702 and the cytoplasmic membrane protein MalF, indicating that these proteins are all inserted into the expected membrane compartment (Fig. 3C and data not shown). The truncated mutant TraDiΔ7-134 shared the fractionation pattern of MalE and LacZ (a periplasmic protein and a cytoplasmic protein, respectively), which was expected, since DiΔ7-134 lacks transmembrane domains (Fig. 3C and data not shown). Degradation products of G-D and TraDiN702, corresponding to the i31-tagged C-terminal TraD cytoplasmic domain, were evident in the soluble material after fractionation, indicating that these proteins are likely cleaved at the membrane after cell lysis. Breakdown products did not appear during coIP studies, which, in contrast to the fractionation experiments, were performed in the presence of protease inhibitors (Fig. 3B, lanes 7 to 8).
To assess the stability of intermolecular TraD interactions, we performed pulse-chase experiments using TraD and TraDiN702 and precipitated proteins as was done for a standard coIP. After a 15-min chase, 75% of both forms of the pulse-labeled TraD remained intact in the cells, indicating significant stability for TraD. The pulse and chase samples both had a ∼1:1 ratio of TraD to TraDiN702 (data not shown), demonstrating that the TraD-TraDiN702 oligomers are stable entities that do not undergo subunit exchange. The stabilities of DiΔ7-134 and G-D were also assessed by pulse-chase analysis and found to be similar to wild-type TraD over a 20-min chase period (data not shown), indicating that the failure of these mutants to interact with wild-type TraD is not due to a rapid degradation of the proteins.
We demonstrated that the associations that we observed by coIP were not postsonication artifacts by performing mixing experiments in which sonicated extracts of strains expressing one TraD::i31 mutant (either K273, E358, or N702) were mixed with the sonicated extract of a strain expressing wild-type TraD prior to immunoprecipitation. A TraD-specific antibody efficiently precipitated both species in a mixed extract, but the i31-specific serum precipitated only the i31 mutant and not wild-type TraD (data not shown), indicating no interaction between the two species. This contrasts with standard coIP experiments in which each of these mutants (coexpressed in the same strain as TraD) showed a robust association with wild-type TraD. Thus, mixing experiments affirm that the coIP method specifically measures the association of TraD monomers in intact membranes, since associations are not observed in vitro in mixed extracts.
Cross-linking of individual TraD mutants reveals intermediates in oligomer assembly.
Given the extensive contacts between C-terminal domains in the TrwBΔN70 crystal structure (16, 17), we had expected that some of our C-terminal i31 insertions would affect monomer associations by sterically preventing favorable intermolecular interactions. However, our coIP results indicated that mutants with C-terminal insertions still interacted with wild-type TraD. We reasoned that in the hexameric TrwBΔN70 structure, each subunit interacted with two neighbors through distinct “faces”; perhaps our mutants had one damaged face but remained competent to interact with wild-type TraD through the undamaged face. Monomers with one damaged interacting face might interact with wild-type TraD but should not be able to associate as closely with each other. To test this hypothesis, we examined TraD oligomerization in cells expressing a single form of TraD (i.e., in cells containing F′ΔD and a vector expressing one traD allele) by DSP cross-linking. DSP is a membrane-permeant cross-linker that reacts with free amines on amino acid residues (predominantly on lysines and at the N terminus) and can be cleaved by reducing agents.
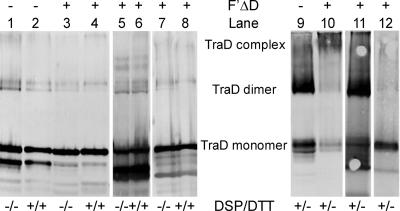
We first cross-linked cells expressing the functional TraDiN702 protein. In these experiments, we qualitatively assessed whole-cell extracts from cross-linked cells on Coomassie-stained SDS-polyacrylamide gels and observed the amount of TraD monomer visible on Western blots at different concentrations of DSP with and without thiol cleavage of the cross-linker. We identified a range of DSP concentrations at which the appearance of TraD was indistinguishable in thiol-cleaved samples and un-cross-linked controls and determined that the optimal concentration of DSP for these studies was 0.1 mM (Fig. 4, lanes 1 to 4). Wild-type TraD and TraDiN702 formed dimer-sized bands in the absence of the F plasmid, indicating homooligomerization. Strikingly, dimers were depleted and these proteins were detected in larger complexes in the presence of the F plasmid (Fig. 4 and data not shown). These results suggest a requirement for F-encoded proteins in the assembly of a functional TraD complex (which may contain other proteins as well).
FIG. 4.
DSP-cross-linked TraD oligomers. TraD species from cross-linking studies were examined after SDS-PAGE and Western blotting. Lanes 1 to 4, 9, and 10, TraDiN702; lanes 5, 6, and 11, TraDiK6; lanes 7, 8, and 12, TraDiK273. The presence or absence of F′ΔD in a strain is indicated by a + or −, respectively, above the lane. DSP cross-linking and dithiothreitol (DTT) cleavage of the cross-linker are indicated as performed (+) or not performed (−) below the lane. Mobilities of TraD monomers, dimers, and larger complexes are shown by the central text. Un-cross-linked, cross-linked, and cleaved samples for each mutant were from a single cross-linking experiment.
We next performed cross-linking experiments with other TraD mutants expressed individually (i.e., in cells expressing no wild-type TraD). TraD mutants were visualized by Western blotting, with representative samples shown in Fig. 4 and with the data summarized in Table 3. TraD::i31 mutants displayed a variety of different behaviors in these experiments, indicating a differential involvement of TraD domains in the oligomerization process. Mutants bearing insertions predicted to disrupt interactions between C-terminal domains based on our structural model of TraD (K273, E358, and A392) failed to form dimer-sized bands or any higher-molecular-weight species. Transfer-deficient mutants with insertions in either the N-terminal cytoplasmic or periplasmic domains (K6 and I59) formed dimer-sized bands but not larger complexes, even in the presence of the F plasmid.
TABLE 3.
Summary of TraD cross-linking results
| Protein | Result
|
|
|---|---|---|
| Cross-links as dimersa | Cross-links as multimersa | |
| TraD | + | + |
| TraDi6 | + | − |
| TraDi59 | + | − |
| TraDi273 | − | − |
| TraDi358 | − | − |
| TraDi392 | − | − |
| TraDi702 | + | + |
Dimer formation for TraD and TraDiN702 was determined in cells lacking the F plasmid, but for other mutants, it was assayed in cells carrying the F plasmid; multimer formation for all mutants was determined in cells carrying the F plasmid. +, detectable; −, not detectable.
Conjugative dominance phenotypes of i31 mutants are consistent with coIP and cross-linking results.
The dominance of several traD::i31 alleles was measured in a conjugation assay (Table 2). It is noteworthy that several of the i31 mutants defective in complementation experiments were also dominant in conjugation, consistent with the ability of mutants such as K273 and A392 to associate with wild-type TraD by coIP. E358 and G408, two defective mutants that coimmunoprecipitated with wild-type TraD, did not display conjugative dominance. However, a Western blot of extracts from the donor cells revealed that the levels of TraDiE358 and TraDiG408 were less than those of wild-type TraD (expressed from F′lac) in the strains used to test conjugative dominance (data not shown), which likely accounts for their recessive phenotype. The truncated cytoplasmic mutants DΔ2-134 and DiΔ7-134 did not display conjugative dominance, and neither did the G-D fusion; unlike TraDiE358 and TraDiG408, these mutants are robustly expressed by donors (data not shown). K6, which was also highly expressed (data not shown), reproducibly showed limited dominance in this assay.
DISCUSSION
In this study, we used a library of i31 mutants to assess the oligomerization of the F plasmid coupling protein TraD. We conclude that TraD monomers form stable associations with one another in the membranes of bacteria. Associations between monomers require sequences within the first 151 residues of the protein and appear to be stabilized by interactions between C-terminal cytoplasmic domains. Our data suggest a back-to-front arrangement of TraD monomers, which is consistent with the model that CPs form ring-shaped functional complexes. Other F-encoded proteins appear to play an important role in the formation of stable, higher-order TraD oligomers.
Previous studies indicated that conjugative CPs can oligomerize in vitro under certain conditions (16, 22, 46, 47), and some evidence suggests an in vivo self-association of the Agrobacterium tumefaciens virulence-associated CP VirD4 (4, 29). We analyzed the process of TraD oligomerization in vivo using coIP, pulse-chase, cross-linking, and genetic techniques. We observed that wild-type TraD and the functional TraDiN702 can associate to form homomeric, and perhaps heteromeric, structures in the inner membrane. Pulse-chase experiments and coIPs demonstrated that these oligomers are stable structures that do not exchange subunits in living cells or solubilized extracts. Oligomerization of the truncated TrwB mutant TrwBΔN70 has been shown to depend upon its binding to single-stranded DNA in vitro (49); working with full-length TraD in vivo, we observed oligomer formation and depletion of monomeric TraD under conditions in which the CP is unlikely to bind single-stranded DNA (i.e., when conjugation is not occurring). We therefore propose that DNA binding is not a prerequisite for CP oligomerization in vivo, although we have yet to demonstrate this conclusively.
It is noteworthy that cross-linking in the presence and absence of the F plasmid revealed differential oligomeric behavior of wild-type TraD (and the functional mutant TraDiN702). Without the F plasmid, a band which appears to be a TraD dimer was clearly visible; with the F plasmid, this band was reduced or absent, and TraD appeared in higher-molecular-weight complexes (often poorly resolved by SDS-PAGE and difficult to transfer onto nitrocellulose for blotting). We therefore believe that TraD can dimerize spontaneously in the membrane but requires other F Tra proteins to assemble into larger stable structures (which may include other proteins bound to TraD). We cannot rule out assembly in the absence of the F plasmid, but the preponderance of TraD dimers in cross-linked extracts of cells lacking the F plasmid indicates that such an assembly is inefficient, forms unstable complexes, or leads to conformationally distinct structures not amenable to cross-linking by DSP.
Our experiments with various mutants show that multiple regions of the TraD protein play distinct roles in oligomer formation. coIP experiments (Fig. 3) demonstrate the importance of the N-terminal transmembrane and hydrophilic domains of the protein by showing that mutants lacking these domains (DΔ2-134, DiΔ7-134, and G-D) cannot bind wild-type TraD in vivo. This result is consistent with the failure of DΔ2-134, DiΔ7-134, and G-D to exhibit conjugative dominance (Table 2 and data not shown). The importance of the N-terminal domains is further attested by previous studies showing that the N-terminally-truncated CP TrwBΔN70 has altered stability and nucleotide-binding ability (23, 24) and that both TrwBΔN70 and N-terminally-truncated mutants of RP4 TraG exhibit monomeric behavior in vitro (22, 47). The K6 and I59 mutants reveal that the N-terminal region of TraD is also important for assembly beyond the dimer stage: these mutants bound wild-type TraD and formed homodimers by cross-linking but did not form the larger cross-linked structures observed for TraD and TraDiN702 even when the F plasmid was present. We conclude that these mutants can form a dimeric intermediate of the TraD oligomer but fail to interact with the F-encoded proteins that normally assemble TraD into larger structures. The limited conjugative dominance of K6 suggests that this mutant is not defective in other transport-related functions, and it may be incorporated into complexes with wild-type TraD without abolishing the activity of the larger assembly.
Although it participates in dimer formation and the assembly of larger structures, the N-terminal region of TraD is not the sole in vivo participant in oligomer formation. Cross-linking experiments highlight the role played by sequences in the C-terminal cytoplasmic domain, since the K273, E358, and A392 mutants expressed by themselves were recovered only as monomers after cross-linking (Fig. 4 and Table 3). It is possible that these mutants do oligomerize through their N termini, but their behavior in our cross-linking assay indicates that they do not form intermolecular contacts as extensively as do TraD and TraDiN702. All three mutants formed heterodimers with wild-type TraD, however. To explain these observations, we examined the predicted locations of their i31 lesions using a structural model of the TraD hexamer covering most of the C-terminal cytoplasmic domain. In this model (Fig. 2), residues 273, 358, and 392 all map near the interface between one monomer and the next. Residue 273 is on one side of the monomer, here designated “face A”; residues 358 and 392 are on the opposite side, “face B.”
The model predicts that each interaction between monomers is an A-B interaction, which explains why mutants with i31 insertions at these positions could interact with wild-type TraD but not themselves in our cross-linking assay. The undamaged B face of TraDiK273 can interact with the A face of wild-type TraD, but this mutant cannot form A-B interactions alone; similarly, TraDiE358 and TraDiA392 interact with wild-type TraD through their functional A faces but do not form A-B interactions by themselves. This interpretation is bolstered by the dominance of the K273 and A392 mutants in conjugation assays (Table 2); E358 did not appear to be dominant, likely due to relatively poor expression in the donor strain (see Results). We conclude that TraD monomers form stable interactions between unlike faces (A-B interactions) rather than like faces (A-A or B-B interactions). Because our data indicate that TraD subunit association relies on A-B interactions, we conclude that TraD oligomers contain a back-to-front arrangement of subunits. A repeated array of back-to-front interactions would become either a long filament or a closed ring; we infer that TraD forms a ring-shaped oligomer in cells.
The TrwBΔN70 crystal structure (17) predicts that CPs form highly symmetrical, ring-shaped homohexamers with a back-to-front arrangement of subunits and extensive interactions along the monomers’ C-terminal cytoplasmic domains. Our findings generally agree with this model. We find that two C-terminal cytoplasmic faces form intermolecular interactions between monomers, and we verify for TraD the back-to-front arrangement predicted for TrwB. Our experiments also allow us to describe aspects of in vivo CP oligomerization not addressed by the crystal structure. We demonstrate the importance of N-terminal sequences not present in the TrwBΔN70 mutant, showing that transmembrane sequences play a role in TraD dimer formation in vivo, and other N-terminal residues are important for the assembly of TraD dimers into larger structures. We show that interactions between TraD and other F-encoded factors (probably proteins) facilitate the assembly of a large and possibly heteromeric complex. The participation of other proteins in in vivo assembly is not surprising, given the role of CPs as a link between substrate processing and secretion modules; CPs have been shown to interact with several cytoplasmic and extracellular T4SS components in both conjugative (5, 11, 12, 14, 18, 19, 21, 35, 36, 46, 49) and virulence (3, 4, 11, 37) models.
Based on the above-described interpretations of our data, we propose the following model to describe the oligomerization of TraD and, by extension, homologous CPs from other T4SSs. TraD monomers are inserted into the membrane and make initial contacts through their N-terminal regions, likely including the transmembrane domains. These interactions are followed by the association of the monomers’ C-terminal cytoplasmic domains, which bind in a back-to-front manner. Another plasmid-encoded protein (or proteins) recognizes the N-terminal sequences of the dimeric complex and helps assemble or stabilize a trimer of TraD dimers into the functional ring-shaped hexamer.
Looking beyond this specific model, our results highlight the structural complexity of molecular motors. TraD represents the simplest class of motor, one composed of identical subunits, and yet it appears to require extensive interactions between monomers along the length of the protein. A fusion protein containing homologous sequences from the RP4 CP TraG could not interact with wild-type TraD despite the fact that these proteins possess highly similar N-terminal domains and identical C-terminal domains. This observation indicates that intermolecular TraD interactions are specific enough to preclude cross talk between closely related proteins.
Furthermore, in vivo oligomerization of TraD required the presence of other F-encoded proteins, implying a layer of regulation which could restrict the activity of TraD and similar motors to their proper spatial and temporal locations. This is an attractive proposition from the point of view of a bacterial cell; a molecular motor operating without its cognate substrate(s) may represent an energy-wasting machine, and so posttranscriptional regulation at the level of assembly might represent a way to minimize futile ATP hydrolysis. In light of these considerations, we find ourselves quite curious about the circumstances in which the larger TraD oligomers are favored over dimers in vivo. We are currently investigating the composition of the larger complexes and the conditions necessary for their formation.
Acknowledgments
This work was supported by the National Science Foundation (MCB-0345018). R. Haft was supported in part by the National Institute of General Medical Sciences (NRSA T32 GM07270). D. Chivian was a National Fellow of the Program in Mathematics and Molecular Biology, with funding from the Burroughs-Wellcome Fund. L. Toussaint was supported by the UW STAR program (T35 HL07763).
We thank John Hodges for generating the TraG-TraD fusion construct and Colin Manoil for his insightful comments and suggestions over the course of this work.
Footnotes
Published ahead of print on 13 July 2007.
REFERENCES
- 1.Achtman, M., N. Willetts, and A. J. Clark. 1971. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J. Bacteriol. 106:529-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. [DOI] [PubMed] [Google Scholar]
- 3.Atmakuri, K., E. Cascales, and P. J. Christie. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54:1199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atmakuri, K., Z. Ding, and P. J. Christie. 2003. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol. Microbiol. 49:1699-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabezón, E., J. I. Sastre, and F. de la Cruz. 1997. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol. Gen. Genet. 254:400-406. [DOI] [PubMed] [Google Scholar]
- 6.Chivian, D., and D. Baker. 2006. Homology modeling using parametric alignment ensemble generation with consensus and energy-based model selection. Nucleic Acids Res. 34:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chivian, D., D. E. Kim, L. Malmstrom, J. Schonbrun, C. A. Rohl, and D. Baker. 2005. Prediction of CASP6 structures using automated Robetta protocols. Proteins 61(Suppl. 7):157-166. [DOI] [PubMed] [Google Scholar]
- 8.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie, P. J. 2004. Type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems. Biochim. Biophys. Acta 1694:219-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Paz, H. D., F. J. Sangari, S. Bolland, J. M. Garcia-Lobo, C. Dehio, F. de la Cruz, and M. Llosa. 2005. Functional interactions between type IV secretion systems involved in DNA transfer and virulence. Microbiology 151:3505-3516. [DOI] [PubMed] [Google Scholar]
- 12.Disque-Kochem, C., and B. Dreiseikelmann. 1997. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J. Bacteriol. 179:6133-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firth, N., K. Ippen-Ihler, and R. A. Skurray. 1996. Structure and function of the F factor and mechanisms of conjugation, p. 2377-2382. In F. C. Neidhardt, R. Curtiss III, C. A. Gross, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 14.Gilmour, M. W., J. E. Gunton, T. D. Lawley, and D. E. Taylor. 2003. Interaction between the IncHI1 plasmid R27 coupling protein and type IV secretion system: TraG associates with the coiled-coil mating pair formation protein TrhB. Mol. Microbiol. 49:105-116. [DOI] [PubMed] [Google Scholar]
- 15.Gomis-Ruth, F. X., F. de la Cruz, and M. Coll. 2002. Structure and role of coupling proteins in conjugal DNA transfer. Res. Microbiol. 153:199-204. [DOI] [PubMed] [Google Scholar]
- 16.Gomis-Ruth, F. X., G. Moncalian, F. de la Cruz, and M. Coll. 2002. Conjugative plasmid protein TrwB, an integral membrane type IV secretion system coupling protein. Detailed structural features and mapping of the active site cleft. J. Biol. Chem. 277:7556-7566. [DOI] [PubMed] [Google Scholar]
- 17.Gomis-Ruth, F. X., G. Moncalian, R. Perez-Luque, A. Gonzalez, E. Cabezón, F. de la Cruz, and M. Coll. 2001. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature 409:637-641. [DOI] [PubMed] [Google Scholar]
- 18.Gunton, J. E., M. W. Gilmour, G. Alonso, and D. E. Taylor. 2005. Subcellular localization and functional domains of the coupling protein, TraG, from IncHI1 plasmid R27. Microbiology 151:3549-3561. [DOI] [PubMed] [Google Scholar]
- 19.Gunton, J. E., M. W. Gilmour, K. P. Baptista, T. D. Lawley, and D. E. Taylor. 2007. Interaction between the co-inherited TraG coupling protein and the TraJ membrane-associated protein of the H-plasmid conjugative DNA transfer system resembles chromosomal DNA translocases. Microbiology 153:428-441. [DOI] [PubMed] [Google Scholar]
- 20.Haft, R. J. F., G. Palacios, T. Nguyen, M. Mally, E. G. Gachelet, E. L. Zechner, and B. Traxler. 2006. General mutagenesis of F plasmid TraI reveals its role in conjugative regulation. J. Bacteriol. 188:6346-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton, C. M., H. Lee, P. L. Li, D. M. Cook, K. R. Piper, S. B. von Bodman, E. Lanka, W. Ream, and S. K. Farrand. 2000. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J. Bacteriol. 182:1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hormaeche, I., I. Alkorta, F. Moro, J. M. Valpuesta, F. M. Goni, and F. De La Cruz. 2002. Purification and properties of TrwB, a hexameric, ATP-binding integral membrane protein essential for R388 plasmid conjugation. J. Biol. Chem. 277:46456-46462. [DOI] [PubMed] [Google Scholar]
- 23.Hormaeche, I., I. Iloro, J. L. Arrondo, F. M. Goni, F. de la Cruz, and I. Alkorta. 2004. Role of the transmembrane domain in the stability of TrwB, an integral protein involved in bacterial conjugation. J. Biol. Chem. 279:10955-10961. [DOI] [PubMed] [Google Scholar]
- 24.Hormaeche, I., R. L. Segura, A. J. Vecino, F. M. Goni, F. de la Cruz, and I. Alkorta. 2006. The transmembrane domain provides nucleotide binding specificity to the bacterial conjugation protein TrwB. FEBS Lett. 580:3075-3082. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey, W., A. Dalke, and K. Schulten. 1996. VMD—Visual Molecular Dynamics. J. Mol. Graphics 14:33-38. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy, K. A., E. G. Gachelet, and B. Traxler. 2004. Evidence for multiple pathways in the assembly of the Escherichia coli maltose transport complex. J. Biol. Chem. 279:33290-33297. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy, K. A., and B. Traxler. 1999. MalK forms a dimer independent of its assembly into the MalFGK2 ATP-binding cassette transporter of Escherichia coli. J. Biol. Chem. 274:6259-6264. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlman, B., and D. Baker. 2000. Native protein sequences are close to optimal for their structures. Proc. Natl. Acad. Sci. USA 97:10383-10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, R. B., and A. Das. 2002. Polar location and functional domains of the Agrobacterium tumefaciens DNA transfer protein VirD4. Mol. Microbiol. 43:1523-1532. [DOI] [PubMed] [Google Scholar]
- 30.Lawley, T. D., B. M. Wilkins, and L. Frost. 2004. Bacterial conjugation in gram-negative bacteria, p. 203-226. In G. Phillips and B. E. Funnell (ed.), Plasmid biology. ASM Press, Washington, DC.
- 31.Lee, M. H., N. Kosuk, J. Bailey, B. Traxler, and C. Manoil. 1999. Analysis of F factor TraD membrane topology by use of gene fusions and trypsin-sensitive insertions. J. Bacteriol. 181:6108-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lessl, M., W. Pansegrau, and E. Lanka. 1992. Relationship of DNA-transfer-systems: essential transfer factors of plasmids RP4, Ti and F share common sequences. Nucleic Acids Res. 20:6099-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippincott, J., and B. Traxler. 1997. MalFGK complex assembly and transport and regulatory characteristics of MalK insertion mutants. J. Bacteriol. 179:1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llosa, M., F. X. Gomis-Ruth, M. Coll, and F. de la Cruz. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 35.Llosa, M., S. Zunzunegui, and F. de la Cruz. 2003. Conjugative coupling proteins interact with cognate and heterologous VirB10-like proteins while exhibiting specificity for cognate relaxosomes. Proc. Natl. Acad. Sci. USA 100:10465-10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, J., and L. S. Frost. 2005. Mutations in the C-terminal region of TraM provide evidence for in vivo TraM-TraD interactions during F-plasmid conjugation. J. Bacteriol. 187:4767-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek, J. A., J. M. Wierzbowski, W. Tao, S. A. Bosak, D. J. Saranga, L. Doucette-Stamm, D. R. Smith, P. J. McEwan, and K. J. McKernan. 2004. Protein interaction mapping on a functional shotgun sequence of Rickettsia sibirica. Nucleic Acids Res. 32:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manoil, C., and J. Bailey. 1997. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J. Mol. Biol. 267:250-263. [DOI] [PubMed] [Google Scholar]
- 39.Manoil, C., and B. Traxler. 2000. Insertion of in-frame sequence tags into proteins using transposons. Methods 20:55-61. [DOI] [PubMed] [Google Scholar]
- 40.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 41.Panagiotidis, C., M. Reyes, A. Sievertsen, W. Boos, and H. Shuman. 1993. Characterization of the structural requirements for assembly and nucleotide binding of an ATP-binding cassette transporter: the maltose transport system of Escherichia coli. J. Biol. Chem. 268:23685-23696. [PubMed] [Google Scholar]
- 42.Panicker, M. M., and E. G. Minkley, Jr. 1985. DNA transfer occurs during a cell surface contact stage of F sex factor-mediated bacterial conjugation. J. Bacteriol. 162:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panicker, M. M., and E. G. Minkley, Jr. 1992. Purification and properties of the F sex factor TraD protein, an inner membrane conjugal transfer protein. J. Biol. Chem. 267:12761-12766. [PubMed] [Google Scholar]
- 44.Rohl, C. A., C. E. Strauss, D. Chivian, and D. Baker. 2004. Modeling structurally variable regions in homologous proteins with Rosetta. Proteins 55:656-677. [DOI] [PubMed] [Google Scholar]
- 45.Sastre, J. I., E. Cabezón, and F. de la Cruz. 1998. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J. Bacteriol. 180:6039-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schröder, G., S. Krause, E. L. Zechner, B. Traxler, H. J. Yeo, R. Lurz, G. Waksman, and E. Lanka. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schröder, G., and E. Lanka. 2003. TraG-like proteins of type IV secretion systems: functional dissection of the multiple activities of TraG (RP4) and TrwB (R388). J. Bacteriol. 185:4371-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons, K. T., C. Kooperberg, E. Huang, and D. Baker. 1997. Assembly of protein tertiary structures from fragments with similar local sequences using simulated annealing and Bayesian scoring functions. J. Mol. Biol. 268:209-225. [DOI] [PubMed] [Google Scholar]
- 49.Tato, I., S. Zunzunegui, F. de la Cruz, and E. Cabezón. 2005. TrwB, the coupling protein involved in DNA transport during bacterial conjugation, is a DNA-dependent ATPase. Proc. Natl. Acad. Sci. USA 102:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Traxler, B., and J. Beckwith. 1992. Assembly of a hetero-oligomeric membrane protein complex. Proc. Natl. Acad. Sci. USA 89:10852-10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waters, C. M., and G. M. Dunny. 2001. Analysis of functional domains of the Enterococcus faecalis pheromone-induced surface protein aggregation substance. J. Bacteriol. 183:5659-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waters, C. M., H. Hirt, J. K. McCormick, P. M. Schlievert, C. L. Wells, and G. M. Dunny. 2004. An amino-terminal domain of Enterococcus faecalis aggregation substance is required for aggregation, bacterial internalization by epithelial cells and binding to lipoteichoic acid. Mol. Microbiol. 52:1159-1171. [DOI] [PubMed] [Google Scholar]