Abstract
Chloroquine-resistant Plasmodium falciparum (CRPF) malaria isolates in Southeast Asia and sub-Saharan Africa share the same Plasmodium falciparum chloroquine resistance transporter (PfCRT) haplotype (CVIET; amino acids 72 to 76). It is believed that CRPF malaria emerged in Southeast Asia and spread to sub-Saharan Africa via the Indian subcontinent. Based on this assumption, we hypothesized that CRPF isolates in India should possess the same drug resistance haplotype (PfCRT haplotype CVIET) as P. falciparum isolates in Southeast Asia and Africa and that the prevalence of CRPF may be higher and more widespread in India than appreciated. To test this postulate, we utilized a standardized real-time PCR assay to assess the prevalence and distribution of PfCRT haplotypes in P. falciparum isolates (n = 406) collected from Western, Central, and Eastern states in India and compared them to isolates from South America and Africa. Based on the proportion of isolates possessing the molecular marker K76T, the prevalence of chloroquine resistance was high in all five regions of India studied (91%), as well as in Uganda (98%) and Suriname (100%). All isolates from Suriname contained the chloroquine-resistant SVMNT haplotype typical of South American isolates, and 98% of isolates from Uganda possessed the chloroquine-resistant CVIET haplotype characteristic of Southeast Asian/African strains. However, of 246 P. falciparum isolates from across India that contained the molecular marker for chloroquine resistance, 81% contained the SVMNT haplotype. In conclusion, the prevalence of CRPF malaria was high in geographically dispersed regions of India, and the primary haplotype observed, SVMNT, did not support a presumed geographic spread from contiguous Southeast Asia.
During the second half of the 20th century, chloroquine (CQ) became the antimalarial agent of choice due to its efficacy, affordability, ease of use, and low toxicity (8). However, the simultaneous appearance of CQ-resistant Plasmodium falciparum malaria in the late 1950s in Southeast Asia and South America and its subsequent spread to most regions to which malaria is endemic have dramatically restricted the usefulness of this drug as a therapeutic or chemoprophylactic agent (28, 30).
CQ resistance in falciparum malaria is associated with mutations in the Pfcrt gene, which encodes the P. falciparum CQ resistance transporter (PfCRT), a transmembrane protein located in the digestive vacuole of malaria parasites (3, 29). A mutation in Pfcrt encoding an amino acid substitution from Lys to Thr at position 76 (K76T) has been shown to be the key molecular marker for CQ resistance, although it most likely confers resistance in concert with other mutations in the same gene (3, 4, 9, 13, 15, 27, 31). The absence of the K76T mutation is highly predictive of CQ sensitivity in vitro and CQ efficacy in vivo (9, 15, 28). Based on the patterns of Pfcrt mutations, haplotype data, and microsatellite analysis demonstrating that CQ-resistant Pfcrt alleles vary depending on geographic origin, CQ resistance is reported to have arisen in at least four independent foci: South America, Southeast Asia, the Philippines, and Papua New Guinea (5, 17, 19, 25, 30, 32).
In general, CQ-resistant isolates from Southeast Asia and Africa (Old World) possess Pfcrt alleles with multiple mutations across PfCRT, corresponding to the amino acid haplotype CVIET (residues 72 to 76), whereas most CQ-resistant parasites from South America (New World) have a SVMNT haplotype (6, 11, 13, 17, 19, 28). Subsequent studies have also demonstrated that Papua New Guinea and the Philippines are also predominantly characterized by a SVMNT haplotype (5, 6, 17, 19). CQ-sensitive strains are characterized by a CVMNK haplotype, regardless of geographic origin.
CQ-resistant P. falciparum (CRPF) malaria isolates in sub-Saharan Africa and Southeast Asia share the CVIET haplotype, and it has been assumed that CRPF parasites spread from Southeast Asia via the Indian subcontinent to the African continent (3, 28, 29, 30). Based on this assumption, we hypothesized that the CRPF isolates in India should possess the CVIET haplotype and that the prevalence of CRPF may be higher than appreciated. However, there are currently limited data available on the distribution and prevalence of CQ resistance and the Pfcrt haplotypes present in P. falciparum isolates from India (2, 10, 18, 23, 26).
In order to examine the prevalence of CRPF, as assessed by a molecular marker of CQ resistance, and to investigate the Pfcrt haplotypes in India, we developed and applied a rapid standardized real-time PCR assay to detect multiple Pfcrt haplotypes associated with CQ resistance in P. falciparum isolates collected from Western, Central, and Eastern states in India. Further, in order to examine the geographic association of PfCRT haplotypes, we compared the haplotypes identified in India with those found in understudied areas of South America (Suriname) and Africa (Uganda) to determine if they fit an “Old World” (CVIET) or “New World” (SVMNT) pattern of geographic origin.
We demonstrate that the prevalence of CQ-resistant P. falciparum malaria as determined by molecular markers of CQ resistance was high (>91%) in the regions of India studied, and the primary haplotype observed, SVMNT, does not appear to support a presumed geographic spread from Southeast Asia.
MATERIALS AND METHODS
Patient selection.
This study, with the aim of detecting and characterizing PfCRT haplotype distribution, was conducted from September 2004 to July 2006 as part of a series of double-blind, randomized clinical trials which investigated the efficacy of CQ plus azithromycin combination therapy in the treatment of P. falciparum infections in Uganda (Kampala and Kakira), Suriname, and India (states of Goa, Maharasthtra, Madhya Pradesh, Orissa, and Assam) (10).
Adults of >18 years of age were enrolled in the study upon meeting eligibility criteria and providing informed consent (10). At each site, the informed consent was translated into the local language. The protocol and the informed consent form were reviewed and approved by the institutional review board at each participating center. Eligibility criteria for participation included documented fever (≥38.5°C) or a history of fever (as reported by the subject) within the prior 24 h, evidence of malaria infection as determined by a rapid diagnostic test, and a confirmatory peripheral-blood smear for malaria with 1,000 to 100,000 parasites/μl on the baseline blood smear. Participants were excluded from the study if they had clinical evidence of severe malaria, including impaired consciousness, jaundice, and/or respiratory distress; were pregnant or lactating; had been treated with any antimalarial drug during the preceding 15 days; and/or had laboratory or clinical evidence of significant abnormality of cardiovascular, liver, or renal function.
Laboratory methods.
Whole blood samples (pretreatment) were collected from all participants for rapid diagnostic testing for P. falciparum (Binax NOW ICT), thick and thin blood film preparation, and subsequent PCR analysis. Thick and thin blood smears were prepared every 8 h until three consecutive smears were negative, and then on day 7 and weekly until the end of the study (day 42). The parasite concentration was determined on the basis of the number of asexual parasites per 200 white blood cells in the thick blood smear. A blood smear was considered negative if no parasites were seen in at least 200 high-power fields.
Real-Time PCR and PfCRT haplotype analysis.
Molecular detection of the K76T mutation associated with CQ resistance was detected by means of a standardized real-time PCR-based diagnostic assay (Artus GmBh; Hamburg, Germany) designed using fluorescent resonance energy transfer technology on a Light Cycler platform (Roche) (12). Briefly, 200 μl of DNA was extracted from whole blood using QIAGEN (Catsworth, CA) columns. Five microliters of extracted DNA was used in the real-time assay to characterize the PfCRT haplotype of each sample by differential melting-curve analysis. Samples clustering around a melting temperature (Tm) of 49 to 51°C represent CQ-sensitive isolates with a CVMNK haplotype, while those clustering around a Tm of 62 to 64°C represent CQ-resistant isolates carrying a CIVET haplotype. The intermediary cluster, with a Tm around 52 to 54°C, identifies isolates with an SVMNT haplotype. A heterologous internal control was included in the assay to monitor both DNA extraction quality and potential PCR inhibition during the real-time PCR run. Furthermore, each run included a known positive control for three PfCRT haplotypes (CQ sensitive, CVMNK; CQ resistant, SVMNT and CVIET). For quality assurance purposes, random samples were selected for direct sequencing to verify the haplotype. Sequencing was performed on an ABI 3730XL instrument.
Nested PCR for malaria species identification.
PCR detection and malaria species identification were performed as described previously (20, 21, 24). Genomic DNA was extracted from whole blood samples using QIAGEN columns (QIAGEN, Catsworth, CA) following the manufacturer's instructions. A 5-μl aliquot of the DNA extract was used in a nested PCR assay to amplify segments of the Plasmodium 18S rRNA gene characteristic for each of the four human malarial species (24). The resulting PCR product was analyzed on a 2% agarose gel stained with ethidium bromide.
RESULTS
A total of 406 participants were diagnosed with P. falciparum infection by microscopy and/or rapid P. falciparum HRP-II antigen detection. Of these, 283 participants were from India, 76 participants were from Suriname, and 47 participants were from Uganda. Pretreatment, whole blood samples from these individuals were coded, processed, and analyzed in a blinded fashion by real-time PCR for Pfcrt mutations. Based on blood smear and antigen detection as the reference standard, 390 of these samples were positive for falciparum malaria by the real-time assay (detecting the P. falciparum Pfcrt gene), for a sensitivity of 96.1% (95% confidence interval, 93.6% to 97.6%). Of the 14 participants from India and 1 participant from Uganda who were classified as having a P. falciparum infection by microscopy and/or antigen detection but were negative for P. falciparum by the real-time PCR assay, nested PCR was carried out to confirm whether the samples were truly negative for malaria or whether the patients were infected with a malaria species other than P. falciparum. Of the 14 samples from India, 11 were confirmed negative for malaria by nested PCR and 3 were identified as Plasmodium vivax infections; and the participant from Uganda was also confirmed negative for P. falciparum malaria by nested PCR assay, indicating a sensitivity of 100% for the real-time PCR assay when using nested PCR as the reference standard.
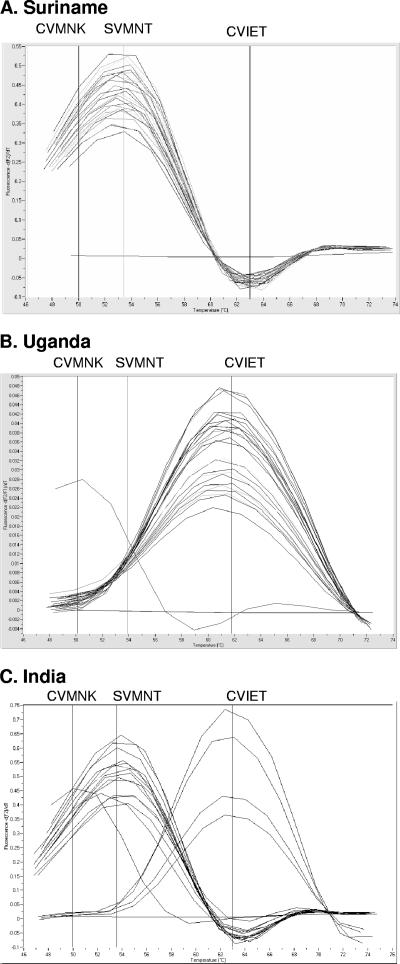
A typical run of the real-time assay for samples from Uganda, Suriname, and India is shown in Fig. 1. Several Pfcrt haplotypes (defined by mutations around positions 72 to 76: S[tct]VMNT, S[agt]VMNT, CVIET, CVMNK) were observed in our patient population. African/Southeast Asian CQ-resistant isolates are characterized by the presence of the CVIET amino acid haplotype, which in the real-time assay occurred at a Tm of ∼63°C. In contrast, CQ-resistant isolates from South America are primarily characterized by the SVMNT haplotype and cluster around a Tm of 54°C (27). All sensitive isolates carried the CVMNK haplotype across positions 72 to 76 and clustered at a Tm of ∼49°C.
FIG. 1.
Representative examples of results obtained with the real-time PCR assay.
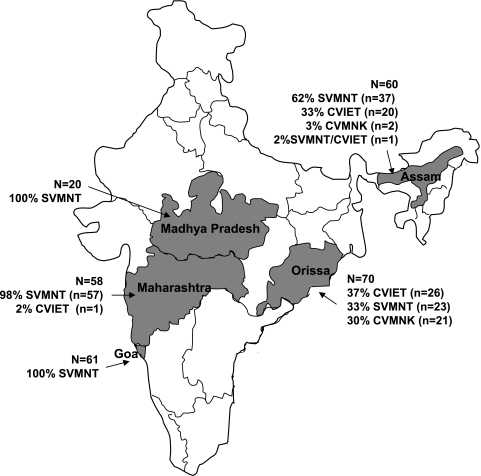
The PfCRT haplotype distribution of CQ-sensitive and CQ-resistant patient-derived isolates, stratified by country, is presented in Table 1. Based on the proportion of P. falciparum isolates possessing the PfCRT molecular marker K76T, the prevalence of CQ resistance was high in all five regions of India studied (91%), as well as being high in Uganda (98%) and Suriname (100%). The geographic distribution and corresponding haplotypes of the Indian P. falciparum isolates are shown in Fig. 2. All 76 P. falciparum isolates from Suriname contained the CQ-resistant SVMNT haplotype typical of South American isolates; and 98% of isolates from Uganda possessed the CQ-resistant CVIET haplotype characteristic of Southeast Asian and African strains. However, of interest, of 245 P. falciparum isolates from across India that contained the molecular marker for CQ resistance, 200 (81%) contained the SVMNT haplotype, typical of South American or Papua New Guinean CQ-resistant isolates, versus that of Southeast Asian and African isolates.
TABLE 1.
Distribution of P. falciparum PfCRT haplotypes
| CQ status | PfCRT haplotype | No. (%) of isolates with haplotype from:
|
||
|---|---|---|---|---|
| India | Uganda | Suriname | ||
| Susceptible | CVMNK | 23 (8.6) | 1 (2) | |
| Resistant | SVMNT | 200 (74) | 76 (100) | |
| CVIET | 45 (17) | 45 (98) | ||
| SVMNT/CVIET mix | 1 (0.4) | |||
| Total | 269 | 46 | 76 | |
FIG. 2.
Geographic origins and haplotype distribution of the Indian sample cohort.
DISCUSSION
The findings of this study are notable for several reasons. First, we utilized a standardized real-time PCR assay to undertake molecular surveillance of drug-resistant malaria and demonstrate that it is a rapid and sensitive assay for detecting and characterizing Pfcrt alleles associated with CQ resistance from geographically diverse sites. The assay detected multiple Pfcrt haplotypes and discriminated between CQ-sensitive and CQ-resistant isolates associated with the CVMNK, CVIET, and SVMNT haplotypes in less than an hour. Since this assay is rapid, automated, standardized, manufactured under GMP, and suitable for use in routine diagnostics laboratories, it possesses advantages over PCR-restriction fragment length polymorphism and/or sequencing strategies to detect Pfcrt allelic types (12). Second, we demonstrate that the prevalence of CQ-resistant falciparum malaria, as determined by the presence of the K76T molecular marker of CQ resistance, is high (>91%) in the three countries studied. This provides molecular resistance surveillance data of public health importance for three relatively understudied regions (14) but is of particular interest in India, where, unlike the case for Suriname and Uganda, CQ had remained a first-line agent for the treatment of falciparum malaria in many regions. High rates of CQ-resistant P. falciparum malaria across broad geographic regions of India may not have previously been fully appreciated or adequately documented (2, 10, 18, 23, 26).
Last, our study adds information relevant to the origin and spread of CQ-resistant malaria in Asia and Africa. In contrast to the presumed evolutionary origin of P. falciparum in Africa (1, 7) and its subsequent spread to Southeast Asia, CQ-resistant malaria is believed to have originated in Southeast Asia and to have subsequently spread to sub-Saharan Africa, arriving in the late 1970s (5, 27-30, 32). Elegant studies evaluating the association of parasite genotypes with geographic origins have demonstrated that South American isolates, as well as those from Papua New Guinea and the Philippines, where CQ resistance has been shown to have emerged independently, are associated with an SVMNT haplotype flanking the K76T mutation. Minor haplotype variations (i.e., CVMNT and CVMET) have also been found in these regions, but their close association with the original SVMNT haplotype has been established (27). In contrast, isolates of Southeast Asian and African origin are characterized by a CVIET haplotype (or infrequent variations thereof, e.g., CVIKT and CVIDT) (3, 16). Our observations from Uganda and Suriname provide additional evidence supporting the association of CVIET and SVMNT haplotypes with isolates of African and South American origin, respectively.
CQ-resistant P. falciparum malaria was first reported for India in 1973 (22). Since then, CQ-resistant P. falciparum malaria has spread and increased in incidence across the Indian subcontinent, making it a significant public health problem (2, 10, 23, 26). However, compared to other regions with significant levels of CQ resistance, India has received relatively limited research attention, especially with respect to the distribution of PfCRT haplotypes. Our study confirms and extends the observations of two recent reports (18, 26) regarding the distribution of Pfcrt alleles among CQ-resistant isolates from India. We used a real-time PCR assay to characterize 269 P. falciparum isolates collected during multicenter clinical trials spanning five Indian states over an 18-month study period. Our results indicate a high prevalence of CQ resistance across the five states examined, associated with mixed allelic types (CVIET and SVMNT) but dominated by an SVMNT (81%) haplotype. Although the existence of mixed haplotypes within a parasite population is not unusual, the predominance of SVMNT in India raises interesting questions regarding the evolution of CQ resistance in this country. The current understanding of the evolution of CQ resistance suggests that a CVIET haplotype emerged in Southeast Asia and spread to Africa (25, 30, 32). Our results from Uganda, as well as those from other studies, demonstrate a 100% association of African CQ-resistant isolates with a CVIET haplotype, thus supporting this evolutionary path. Considering India's geographic location, it has been assumed (30) that CQ resistance in India would have been seeded from Southeast Asia and would consequently be characterized by a CVIET haplotype. Eighteen percent of the isolates analyzed do, in fact, carry a CVIET haplotype and may have been introduced from Southeast Asia before being spread to Africa, but the predominance of the SVMNT haplotype in India, typically associated with New World (i.e., South American) or Papua New Guinea isolates, appears to challenge this assumption. Although importation of parasites containing the CQ-resistant haplotype from South America, Papua New Guinea, or the Philippines is possible, it would appear unlikely that isolated introduction(s) of the SVMNT haplotype by visitors or immigrants to India would have resulted in such widespread dissemination of this haplotype over the CVIET type unless it offered some additional selective advantage.
Determining whether the SVMNT haplotype was introduced or evolved independently in India will require additional study. A multilocus microsatellite analysis comparing the evolutionary proximity of the Indian SVMNT haplotype with those of South America (SVMNT/HSQDLR) (32), Papua New Guinea (SVMNT/HLQDLR) (5, 32), and the Philippines (SVMNT/HLQDR or SVMNT/HLQNR) (5) might provide evidence of evolutionary similarity and thus would support an introduction of the SVMNT haplotype from these regions, or conversely, it would support the possibility of an independent focus of CQ resistance emergence in India.
Recombination of de novo point mutations transforming the CVIET haplotype into an SVMNT haplotype have been previously suggested in the context of CQ resistance in Papua New Guinea (17). Such genetic recombination, perhaps driven by continued drug pressure, may confer a selective advantage on parasites possessing the SVMNT haplotype over the CVIET haplotype. Genetic marker analyses at inter- or intrachromosomal regions would reveal whether such recombination events have occurred in Indian isolates.
In summary, using real-time PCR detection and characterization of PfCRT haplotypes, we demonstrate a high prevalence of CQ resistance in multiple sites in India, which has implications for malaria treatment policies. The available evidence suggests that CQ resistance has arisen independently in multiple locations, generating similar PfCRT haplotypes in isolates from distinct geographic regions (5, 17, 25, 26, 32).
Acknowledgments
We are indebted to the following study site investigators, without whom this study would not have been possible: Nilima Kshirsagar, Neeru Singh, Manmohan Shukla, Neena Valecha, P. C. Bhattacharyya, Vas Dev, Kanta Patel, Manoj K. Mohapatra, Jitendra Lakhani, Stephen Vreden, Gregory Utz, Peter Mugyenyi, Saroj Mishra, Nagesh Dubhashi, Rajesh Gosavi, and Ashok Sethia.
With respect to perceived conflicts of interest related to this article, M.W.D. is employed by Pfizer and provided samples for evaluation. J.K., G.A.F., K.Z., S.Y., and K.C.K. have no conflict of interest in association with this article.
This work was supported by the CIHR Team Grant in Malaria (K.C.K.), operating grant MT-13721 (K.C.K.), Genome Canada through the Ontario Genomics Institute (K.C.K.), PSI (K.C.K.), and the CIHR Canada Research Chair program (K.C.K.).
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Anderson, T. J., B. Haubold, J. T. Williams, J. G. Estrada-Franco, L. Richardson, R. Mollinedo, M. Bockarie, J. Mokili, S. Mharakurwa, N. French, J. Whitworth, I. D. Velez, A. H. Brockman, F. Nosten, M. U. Ferreira, and K. P. Day. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 17:1467-1482. [DOI] [PubMed] [Google Scholar]
- 2.Attaran, A., K. I. Barnes, R. Bate, F. Binka, U. d'Alessandro, C. I. Fanello, L. Garrett, T. K. Mutabingwa, D. Roberts, C. H. Sibley, A. Talisuna, J. P. Van Geertruyden, and W. M. Watkins. 2006. The World Bank: false financial and statistical accounts and medical malpractice in malaria treatment. Lancet 368:247-252. [DOI] [PubMed] [Google Scholar]
- 3.Bray, P. G., R. E. Martin, L. Tilley, S. A. Ward, K. Kirk, and D. A. Fidock. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 56:323-333. [DOI] [PubMed] [Google Scholar]
- 4.Carlton, J. M., D. A. Fidock, A. Djimde, C. V. Plowe, and T. E. Wellems. 2001. Conservation of a novel vacuolar transporter in Plasmodium species and its central role in chloroquine resistance of P. falciparum. Curr. Opin. Microbiol. 4:415-420. [DOI] [PubMed] [Google Scholar]
- 5.Chen, N., D. W. Wilson, C. Pasay, D. Bell, L. B. Martin, D. Kyle, and Q. Cheng. 2005. Origin and dissemination of chloroquine-resistant Plasmodium falciparum with mutant pfcrt alleles in the Philippines. Antimicrob. Agents Chemother. 49:2102-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, N., D. E. Kyle, C. Pasay, E. V. Fowler, J. Baker, J. M. Peters, and Q. Cheng. 2003. pfcrt allelic types with two novel amino acid mutations in chloroquine-resistant Plasmodium falciparum isolates from the Philippines. Antimicrob. Agents Chemother. 47:3500-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway, D. J., C. Fanello, J. M. Lloyd, B. M. Al-Joubori, A. H. Baloch, S. D. Somanath, C. Roper, A. M. Oduola, B. Mulder, M. M. Povoa, B. Singh, and A. W. Thomas. 2000. Origin of Plasmodium falciparum malaria is traced by mitochondrial DNA. Mol. Biochem. Parasitol. 111:163-171. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, R. A., C. L. Hartwig, and M. T. Ferdig. 2005. pfcrt is more than the Plasmodium falciparum chloroquine resistance gene: a functional and evolutionary perspective. Acta Trop. 94:170-180. [DOI] [PubMed] [Google Scholar]
- 9.Djimde, A., O. K. Doumbo, J. F. Cortese, K. Kayentao, S. Doumbo, Y. Diourte, A. Dicko, X. Z. Su, T. Nomura, D. A. Fidock, T. E. Wellems, C. V. Plowe, and D. Coulibaly. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257-263. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, M. W., N. Singh, M. Shukla, N. Valecha, P. C. Bhattacharyya, V. Dev, K. Patel, M. K. Mohapatra, J. Lakhani, R. Benner, C. Lele, and K. Patki. 2005. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J. Infect. Dis. 191:1582-1588. [DOI] [PubMed] [Google Scholar]
- 11.Durrand, V., A. Berry, R. Sem, P. Glaziou, J. Beaudou, and T. Fandeur. 2004. Variations in the sequence and expression of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) and their relationship to chloroquine resistance in vitro. Mol. Biochem. Parasitol. 136:273-285. [DOI] [PubMed] [Google Scholar]
- 12.Farcas, G. A., R. Soeller, K. Zhong, A. Zahirieh, and K. C. Kain. 2006. Real-time PCR assay for the rapid detection and characterization of chloroquine-resistant Plasmodium falciparum malaria in returned travelers. Clin. Infect. Dis. 42:622-627. [DOI] [PubMed] [Google Scholar]
- 13.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, M. T. Ferdig, L. M. Ursos, A. B. Sidhu, B. Naude, K. W. Deitsch, X. Z. Su, J. C. Wootton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamya, M. R., N. N. Bakyaita, A. O. Talisuna, W. M. Were, and S. G. Staedke. 2002. Increasing antimalarial drug resistance in Uganda and revision of the national drug policy. Trop. Med. Int. Health. 7:1031-1041. [DOI] [PubMed] [Google Scholar]
- 15.Kublin, J. G., J. F. Cortese, E. M. Njunju, R. A. Mukadam, J. J. Wirima, P. N. Kazembe, A. A. Djimde, B. Kouriba, T. E. Taylor, and C. V. Plowe. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 187:1870-1875. [DOI] [PubMed] [Google Scholar]
- 16.Lim, P., S. Chy, F. Ariey, S. Incardona, P. Chim, R. Sem, M. B. Denis, S. Hewitt, S. Hoyer, D. Socheat, O. Merecreau-Puijalon, and T. Fandeur. 2003. pfcrt polymorphism and chloroquine resistance in Plasmodium falciparum strains isolated in Cambodia. Antimicrob. Agents Chemother. 47:87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlotra, R. K., H. Fujioka, P. D. Roepe, O. Janneh, L. M. Ursos, V. Jacobs-Lorena, D. T. McNamara, M. J. Bockarie, J. W. Kazura, D. E. Kyle, D. A. Fidock, and P. A. Zimmerman. 2001. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc. Natl. Acad. Sci. USA 98:12689-12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittra, P., S. Vinayak, H. Chandawat, M. K. Das, N. Singh, S. Biswas, V. Dev, A. Kumar, M. A. Ansari, and Y. D. Sharma. 2006. Progressive increase in point mutations associated with chloroquine resistance in Plasmodium falciparum isolates from India. J. Infect. Dis. 193:1304-1312. [DOI] [PubMed] [Google Scholar]
- 19.Nagesha, H. S., G. J. Casey, K. H. Rieckmann, D. J. Fryauff, B. S. Laksana, J. C. Reeder, J. D. Maguire, and J. K. Baird. 2003. New haplotypes of the Plasmodium falciparum resistance transporter (pfcrt) gene among chloroquine-resistant parasite isolates. Am. J. Trop. Med. Hyg. 68:398-402. [PubMed] [Google Scholar]
- 20.Pieroni, P., C. D. Mills, C. Ohrt, M. A. Harrington, and K. C. Kain. 1998. Comparison of the ParaSight-F test and the ICT Malaria Pf test with the polymerase chain reaction for the diagnosis of Plasmodium falciparum malaria in travellers. Trans. R. Soc. Trop. Med. Hyg. 92:166-169. [DOI] [PubMed] [Google Scholar]
- 21.Pillai, D. R., A. C. Labbe, V. Vanisaveth, B. Hongvangthong, S. Pomphida, S. Inkathone, K. Zhong, and K. C. Kain. 2001. Plasmodium falciparum malaria in Laos: chloroquine treatment outcome and predictive value of molecular markers. J. Infect. Dis. 183:789-795. [DOI] [PubMed] [Google Scholar]
- 22.Sharma, V. P. 1996. Re-emergence of malaria in India. Indian J. Med. Res. 103:26-45. [PubMed] [Google Scholar]
- 23.Sharma, Y. D., S. Biswas, C. R. Pillai, M. A. Ansari, T. Adak, and C. U. Devi. 1996. High prevalence of chloroquine resistant Plasmodium falciparum infection in Rajasthan epidemic. Acta Trop. 62:135-141. [DOI] [PubMed] [Google Scholar]
- 24.Snounou, G., S. Viriyakosol, X. P. Zhu, W. Jarra, L. Pinheiro, V. E. do Rosario, S. Thaithong, and K. N. Brown. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315-320. [DOI] [PubMed] [Google Scholar]
- 25.Su, X. Z., L. A. Kirkman, H. Fujioka, and T. E. Wellems. 1997. Complex polymorphisms in a ∼330kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell 91:593-603. [DOI] [PubMed] [Google Scholar]
- 26.Vathsala, P. G., A. Pramanik, S. Dhanasekaran, C. U. Devi, C. R. Pillai, S. K. Subbarao, S. K. Ghosh, S. N. Tiwari, T. S. Sathyanarayan, P. R. Deshpande, G. C. Mishra, M. R. Ranjit, A. P. Dash, P. N. Rangarajan, and G. Padmanaban. 2004. Widespread occurrence of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) gene haplotype SVMNT in P. falciparum malaria in India. Am. J. Trop. Med. Hyg. 70:256-359. [PubMed] [Google Scholar]
- 27.Vieira, P. P., M. U. Ferreira, M. G. Alecrim, W. D. Alecrim, L. H. da Silva, M. M. Sihuincha, D. A. Joy, J. Mu, X. Z. Su, and M. G. Zalis. 2004. pfcrt polymorphism and the spread of chloroquine resistance in Plasmodium falciparum populations across the Amazon Basin. J. Infect. Dis. 190:417-424. [DOI] [PubMed] [Google Scholar]
- 28.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]
- 29.Wellems, T. E. 2002. Plasmodium chloroquine resistance and the search for a replacement anti-malarial drug. Science 298:124-126. [DOI] [PubMed] [Google Scholar]
- 30.Wellems, T. E. 2004. Transporter of a malaria catastrophe. Nat. Med. 10:1169-1171. [DOI] [PubMed] [Google Scholar]
- 31.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]
- 32.Wootton, J. C., X. Feng, M. T. Ferdig, R. A. Cooper, J. Mu, D. I. Baruch, A. J. Magill, and X. Z. Su. 2002. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 418:320-323. [DOI] [PubMed] [Google Scholar]