Abstract
Bortezomib, an inhibitor of the 26S proteasome, is currently approved for treatment of multiple myeloma and is being studied for therapy of non-Hodgkin's lymphoma. We found that Epstein-Barr virus (EBV)-positive B cells with type III latency were more susceptible to killing by bortezomib than those with type I latency. Bortezomib induced apoptosis of EBV lymphoblastoid cell lines (LCLs) by inducing cleavage of caspases 8 and 9; apoptosis was inhibited by pretreatment with a pan-caspase inhibitor. Bortezomib reduced the levels of the p50 and p65 components of the canonical NF-κB pathway and reduced the level of p52 in the noncanonical NF-κB pathway, which is induced by EBV LMP1. Bortezomib inhibited expression of cIAP-1, cIAP-2, and XIAP, which are regulated by NF-κB and function as inhibitors of apoptosis. Bortezomib did not inhibit expression of several other antiapoptotic proteins, including Bcl-2 and Bcl-XL. Finally, bortezomib significantly prolonged the survival of severe combined immunodeficiency mice inoculated with LCLs. These findings suggest that bortezomib may represent a novel strategy for the treatment of certain EBV-associated lymphomas.
Epstein-Barr virus (EBV) infects over 95% of the world's population and is associated with several human malignancies, including Hodgkin's disease, Burkitt lymphoma, and nasopharyngeal carcinoma. EBV-associated lymphoproliferative disease occurs in immunosuppressed persons, such as transplant recipients, AIDS patients, and persons with congenital immunodeficiencies (12). EBV plays an important role in the pathogeneses of many of these malignancies because of its ability to establish latent infection and induce proliferation of infected B cells (28). EBV gene expression differs among malignancies associated with the virus. EBV-positive Burkitt lymphoma tissues usually have a type I latency pattern with expression of EBNA-1 but not the other latency-associated proteins (29). Tissues from patients with Hodgkin's disease, nasopharyngeal carcinoma, and T-cell lymphomas usually have a type II latency pattern with expression of EBNA-1, LMP1, and LMP2. Tissues from immunocompromised patients with EBV lymphoproliferative disease generally have a type III latency pattern and express each of the nine latency-associated proteins. Although the treatment for some EBV-associated malignancies has improved in recent years, newer approaches to therapy are needed.
Inhibition of nuclear factor kappa B (NF-κB) is a therapeutic target for inducing apoptosis of a variety of tumor cells (13, 40). The NF-κB family of proteins consists of five members: c-Rel, p65/RelA, RelB, p50/p105 (NF-κB1), and p52/p100 (NF-κB2) (5). NF-κB can be activated by a variety of stimuli, including interleukin-1 and tumor necrosis factor alpha, and among its multiple effects, it induces antiapoptotic proteins and promotes cell survival (17). There are two NF-κB pathways, the canonical and the noncanonical pathways. In the canonical NF-κB pathway, NF-κB is retained in the cytosol by its interaction with IκBs (IκBα, IκBβ, IκBɛ). Following activation, IκBs are phosphorylated by IκB kinases and subsequently degraded by the ubiquitin-proteasome pathway (38); liberated NF-κB translocates to the nucleus, where it activates gene expression. In the noncanonical NF-κB pathway, phosphorylation of p100 with subsequent ubiquitination and proteasome-mediated processing yields p52, which together with RelB translocates to the nucleus to activate transcription (44). Both pathways can promote tumor development. In many cancers, NF-κB is constitutively activated, which protects tumor cells from apoptosis.
Infection of resting human B lymphocytes in vitro with EBV results in expression of LMP1, with constitutive activation of NF-κB, B-cell proliferation, and transformation of the cells to lymphoblastoid cell lines (LCLs). LMP1 acts as a functional homolog of a constitutively active form of CD40 and activates both the canonical and the noncanonical NF-κB pathways (4, 18, 32, 37). Lesions from patients with EBV-associated B-cell lymphomas and posttransplant lymphoproliferative disease show activation of NF-κB, and LMP1 colocalizes with TRAF-1 and TRAF-3 (31, 39).
Inhibition of NF-κB (e.g., by transfection with an IκBα mutant or treatment with Bay 11-7082 or simvastatin) induces apoptosis of EBV-transformed B cells (9, 10, 25). Bortezomib (PS-341) is an inhibitor of the 26S proteasome (1). Proteasomes are multiprotein complexes that degrade ubiquitinated proteins, including those involved in cell cycle regulation, oncogenesis, and apoptosis. Inhibition of the proteasome can result in apoptosis of malignant cells (36, 50). Bortezomib has many activities, including inhibition of IκBα degradation with subsequent inhibition of constitutive NF-κB, which results in apoptosis of cells (22). Bortezomib is approved for the treatment of multiple myeloma and is in clinical trials for non-Hodgkin's lymphoma, prostate cancer, and lung cancer (24, 41).
Here, we investigate the effect of bortezomib on EBV-transformed B cells both in vitro and in vivo. We show that bortezomib induces apoptosis of the cells through caspase-dependent pathways in vitro and inhibits p52, cellular inhibitor-of-apoptosis protein 1 (cIAP-1), c-IAP-2, and X-chromosome-linked inhibitor-of-apoptosis protein (XIAP) expression. Bortezomib inhibits development of EBV B-cell lymphomas in severe combined immunodeficiency (SCID) mice. These findings suggest that bortezomib may serve as a novel therapy for the treatment of certain EBV-associated lymphomas.
MATERIALS AND METHODS
Cell lines and reagents.
Four EBV-transformed LCLs (clones a, b, Bra, and Der), EBV-positive Burkitt lymphoma cell lines (Akata [45], Mutu I, and Mutu III [21]), and EBV-negative Burkitt lymphoma cell lines (BJAB [33], EBV-negative Akata cells) were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, penicillin, and streptomycin. 293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and penicillin-streptomycin. A BJAB vector control (BJgpt-3) and BJAB cells stably expressing LMP1 (BJLMP-6 and BJLMP-15) were a gift from Fred Wang (Harvard University) and were grown in media containing mycophenolic acid, xanthine, and hypoxanthine as previously described (48). Bortezomib (Millennium Pharmaceutical Inc., Cambridge, MA) was dissolved in phosphate-buffered saline (PBS), and the pan-caspase inhibitor Q-VD-OPH (R&D Systems, Minneapolis, MN) and Bay 11-7082 (Calbiochem, La Jolla, CA) were dissolved in dimethyl sulfoxide.
Cell viability and apoptosis assays.
Cells (2 ×105 per ml) were cultured in 12-well plates for 1 day, and cell viability was quantified using trypan blue exclusion with a Vi-Cell cell viability analyzer (Beckman Coulter, Fullerton, CA). The pan-caspase inhibitor Q-VD-OPH (11) was added at 50 μM 1 h prior to addition of bortezomib.
Viable LCLs were isolated using Ficoll-Paque (Amersham Pharmacia Biotech AB, Uppsala, Sweden) gradient centrifugation. Apoptosis was measured by flow cytometry using an annexin V-phycoerythrin (PE)/7-aminoactinomycin D (7-AAD) apoptosis assay kit (BD Pharmingen Biosciences, San Diego, CA) according to the manufacturer's instructions. Briefly, 2 × 105 LCLs were treated with various concentrations of bortezomib and after 6 h washed in ice-cold PBS, resuspended in binding buffer, incubated with annexin V-PE for 15 min, and analyzed by flow cytometry. Viable LCLs were defined as negative for annexin V-PE and 7-AAD staining, and apoptotic LCLs were defined as positive for annexin V-PE and negative for 7-AAD staining.
Immunoblots.
Whole-cell extracts were prepared after lysis in extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, and protease inhibitors), and tissue extracts were prepared by homogenizing tissue in extraction buffer. Protein concentrations were determined with a Micro bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL). Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and immunoblotted with antibodies. Antibodies were directed against caspase 3, cleaved caspase 3, caspase 7, caspase 8, cleaved caspase 8, caspase 9, IκBα, phosphorylated IκBα, and polyadenosine diphosphate-ribose polymerase (PARP) (Cell Signaling Technology, Beverly, MA); β-actin (Sigma, St. Louis, MO); Bcl-2 (Oncogene, Cambridge, MA); Bax (Trevigene, Gaithersburg, MD); XIAP, cIAP-1, and cIAP-2 (R&D Systems, Minneapolis, MN); p52 and Bcl-XL (Santa Cruz Biotech Inc., Santa Cruz, CA); TRAF2 (BD Pharmingen, San Diego, CA); BZLF-1 and LMP1 (DAKO, Carpintera, CA); and EBV early antigen-D (EA-D) (Capricorn, Portland, ME).
Electrophoretic mobility shift assays.
Nuclear extracts were prepared using a nuclear-extract kit (Active Motif, Carlsbad, CA) according to the manufacturer's instructions. Activation of NF-κB was determined by using 5 μg of nuclear extracts in a gel shift assay system (Promega, Madison, WI) according to the manufacturer's instructions. Nuclear protein extracts were incubated with a 32P-labeled oligonucleotide containing a conserved NF-κB binding sequence, and electrophoretic mobility shift assays were performed. Nonradiolabeled specific or nonspecific competitor oligonucleotides were added in some assays. Antibodies to human p50 (6) or p65 (19) were added in some experiments. Quantitative evaluation of NF-κB complex formation was determined using a Storm 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA) with ImageQuant software.
Animal experiments.
SCID mice (4 to 6 weeks old) were inoculated intraperitoneally (i.p.) with 0.5 × 106 or 1 × 106 LCLs, and 7 days later, 0.5 mg of bortezomib/kg of body weight or PBS was given i.p. three times a week for 6 weeks. Mice were monitored daily for survival, and all dead mice were autopsied and examined for the presence of lymphomas.
RESULTS
EBV-infected B cells with a type III latency pattern are highly susceptible to killing by bortezomib.
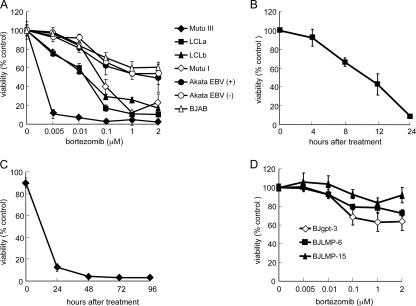
To determine the effects of bortezomib on different EBV-positive B-cell lines, we treated the cells with various concentrations of bortezomib. One day after treatment, cell viability assays were performed. Bortezomib reduced the number of viable cells in a dose-dependent manner (Fig. 1A). EBV-positive B cells with a type III latency pattern (LCLa, LCLb, and Mutu III cells) were more sensitive to killing by bortezomib than EBV-positive B cells with a type I latency pattern (Mutu I and EBV-positive Akata cells) or EBV-negative B cells (BJAB and EBV-negative Akata cells). LCLs were rapidly killed by bortezomib, with 50% of the cells killed within 12 h of treatment with the drug (Fig. 1B). Cells treated with bortezomib for four consecutive days showed a persistent loss in viability over time (Fig. 1C). Thus, bortezomib preferentially killed EBV-positive B cells with a latency III pattern.
FIG. 1.
Bortezomib induces death of LCLs. (A) Cells were treated with various doses of bortezomib for 24 h, and viability was measured by trypan blue staining. Percent viability was defined as mean numbers of viable cells in treated versus untreated cells. Each point represents the mean ± standard deviation (bar) for triplicate experiments. (B, C) LCLs were treated with bortezomib (1 μM) for the indicated time periods. Cell viability was performed as described for panel A. (D) BJgpt-3, BJLMP-6, and BJLMP-15 cells were treated with various concentrations of bortezomib for 24 h, and viability was measured as described for panel A.
Since LMP1 is expressed in cells with a type III EBV latency pattern but not in cells with a type I latency pattern, we tested whether BJAB cells stably expressing LMP1 (BJLMP-6 or BJLMP-15) were more susceptible to killing by bortezomib than BJAB cells transfected with the control vector (BJgpt-3). Treatment of BJAB cells expressing LMP1 showed no increase in sensitivity to bortezomib compared with treatment of vector control cells (Fig. 1D). This suggests that LMP1 alone is not responsible for the increased susceptibility of cells with a latency type III pattern to bortezomib.
Bortezomib induces apoptosis of LCLs.
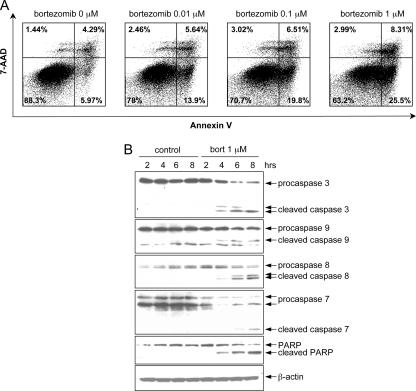
To investigate whether bortezomib kills LCLs by inducing apoptosis, we treated cells with various concentrations of bortezomib for 6 h and apoptosis was analyzed by flow cytometry after annexin V staining. LCLa cells treated with bortezomib showed increasing numbers of apoptotic (annexin V-positive, 7-AAD-negative) cells in a dose-dependent manner compared to untreated cells (Fig. 2A). To further analyze the mechanism of bortezomib-induced apoptosis, caspase and PARP cleavage—hallmarks of apoptosis—were investigated by immunoblotting. Bortezomib induced cleavage of caspases 3, 7, 8, and 9 and PARP in LCLa cells; increased cleavage occurred with longer periods of exposure to bortezomib (Fig. 2B).
FIG. 2.
Bortezomib induces apoptosis of LCLs. (A) LCLa cells were incubated with various concentrations of bortezomib, and apoptosis was evaluated 6 h later by annexin V-7-AAD staining. The percentage of annexin-positive, 7-AAD-negative cells is indicated on each panel. (B) Apoptosis induced by bortezomib is associated with cleavage of caspases 3, 7, 8, and 9 and PARP. Viable LCLa cells were incubated with 1 μM of bortezomib (bort) for different periods of time, protein lysates were prepared, and immunoblotting was performed. β-Actin was used as a loading control.
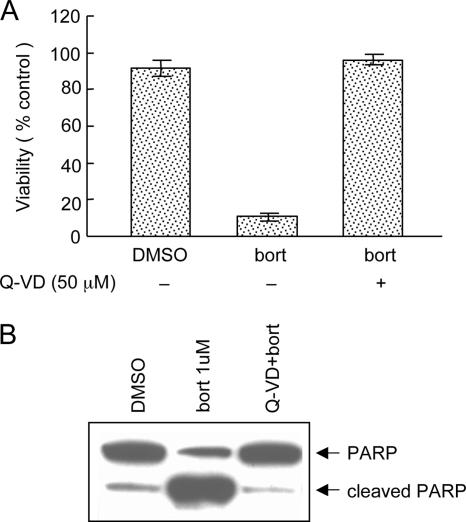
To determine whether inhibition of caspases would reduce apoptosis induced by bortezomib, LCLa cells were incubated with Q-VD-OPH (a pan-caspase inhibitor) for 1 h, followed by treatment with bortezomib (1 μM) for 24 h. Pretreatment with Q-VD-OPH completely blocked bortezomib-induced cell death (Fig. 3A) and PARP cleavage (Fig. 3B). Similar results were observed in LCLb cells treated with Q-VD-OPH (data not shown). Thus, bortezomib induced apoptosis in LCLs, which was blocked by a pan-caspase inhibitor.
FIG. 3.
Q-VD-OPH inhibits bortezomib-induced apoptosis. LCLa cells were incubated with Q-VD-OPH (50 μM) for 1 h followed by bortezomib (bort) (1 μM) for 24 h. (A) Cell viability was assessed as for Fig. 1A. Error bars represent standard deviations. (B) PARP cleavage was determined as for Fig. 1B after the cells were treated with Q-VD-OPH for 1 h followed by bortezomib.
Bortezomib inhibits NF-κB activity and expression of p52.
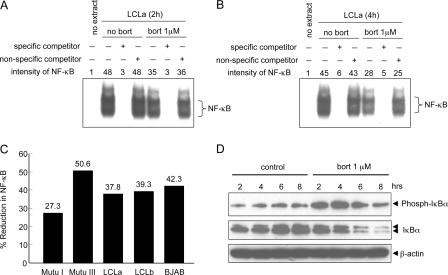
EBV LMP1 can induce NF-κB activation by the canonical and noncanonical pathways, which can lead to inhibition of apoptosis (4, 18, 32, 37). Treatment of LCLa cells with bortezomib led to a modest inhibition of NF-κB DNA-binding activity after 2 h (Fig. 4A) or 4 h (Fig. 4B) in electrophoretic mobility shift assays. Incubation with an excess of nonradioactive-specific competitor oligonucleotide blocked binding of NF-κB to DNA, while a nonspecific oligonucleotide did not block binding. Bortezomib induced a reduction of NF-κB in all of the EBV-positive and EBV-negative cell lines tested. The inhibition of NF-κB ranged from 27% to 51% in cells treated with bortezomib for 4 h relative to the level in untreated cells (Fig. 4C). In contrast, bortezomib inhibited Oct1 by only 16% in LCLb cells and 18% in BJAB cells (data not shown). Treatment of LCLa cells with bortezomib increased the phosphorylation of IκBα and decreased the level of total IκBα (Fig. 4D).
FIG. 4.
Bortezomib inhibits NF-κB activity in LCLs. LCLa cells were cultured in the absence or presence of 1μM bortezomib for 2 h (A) or 4 h (B), and nuclear extracts were used in electrophoretic mobility shift assays with a radiolabeled NF-κB probe. Specific or nonspecific nonradioactive competitor oligonucleotides were added in some assays. (C) Mutu I, Mutu III, LCLa, LCLb, and BJAB cells were analyzed by electrophoretic mobility shift assays for NF-κB as described for panel B, and the reduction of NF-κB in cells treated with bortezomib for 4 h relative to the level in untreated cells is shown. Data for LCLa was derived from panel B. (D) LCLa cells were incubated in the absence or presence of 1 μM bortezomib (bort) for the time periods indicated above each lane, and proteins from whole-cell extracts were immunoblotted with anti-phosphorylated IκBα and anti-IκBα antibodies. Anti-β-actin antibody was used as a protein loading control.
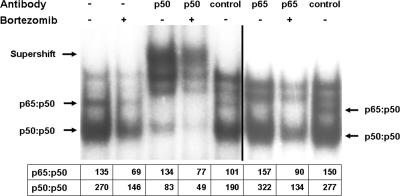
To determine which components of NF-κB were inhibited by bortezomib, electrophoretic mobility shift assays were performed in the presence of antibodies to p50 and p65. Antibodies to p50 or p65 resulted in shifts of the corresponding proteins (Fig. 5). Quantification of the level of p50 homodimer in LCLa cells by use of antibody to p50 showed that the level was reduced by 41% in cells treated with bortezomib compared with that in untreated cells. The level of p65:p50 heterodimer quantified using antibody to p65 was reduced by 43% in cells treated with bortezomib compared to the level in untreated cells.
FIG. 5.
Bortezomib inhibits activation of p50 and p65. LCLa cells were cultured in the absence or presence of 1 μM bortezomib for 4 h, nuclear extracts were prepared, and electrophoretic mobility shift assays were performed with a radiolabeled NF-κB probe in the presence of preimmune rabbit sera or rabbit antibody to p50 or p65. Numbers below the autoradiogram show intensities of p65:p50 heterodimer and p50:p50 homodimer bands assayed by densitometry.
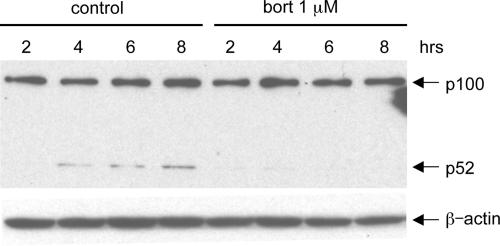
The noncanonical NF-κB activation pathway is induced in EBV-transformed B cells and involves proteasome-mediated processing of the NF-κB precursor p100 into active p52. Bortezomib significantly reduced expression of p52 in LCLa cells (Fig. 6). Similar results were obtained with LCLb and LCL Der cells (data not shown). Thus, these data suggest that bortezomib can inhibit the canonical and noncanonical NF-κB pathways in LCLs.
FIG. 6.
Bortezomib inhibits expression of p52. LCLa cells were treated with or without 1 μM bortezomib (bort) for the indicated times, and whole-cell lysates were immunoblotted with anti-p52 antibody to analyze the expression of p100 and p52. β-Actin was used as a control for protein loading.
Bortezomib down-regulates expression of cIAP-1, cIAP-2, and XIAP in LCLs.
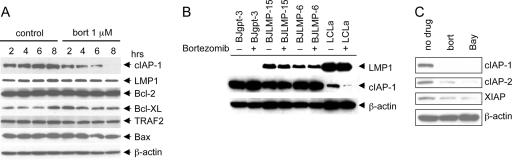
NF-κB up-regulates expression of several antiapoptotic proteins, such as cIAP-1, cIAP-2, and TRAFs, and blocks apoptosis directly at the step of caspase activation (3, 47). Since induction of apoptosis by bortezomib was associated with inhibition of NF-κB in LCLs, we tested whether bortezomib reduced the expression of antiapoptotic proteins. Bortezomib reduced the expression of cIAP-1 in LCLa cells as early as 4 h after treatment was begun, and the protein was nearly absent at 8 h (Fig. 7A). In contrast, no reduction in expression of cIAP-1 was noted in LMP1-expressing BJAB cells (BJLMP-6 or BJLMP-15) treated with bortezomib, compared to the level in control vector-containing BJAB cells (BJgpt-3) treated with the drug (Fig. 7B). This suggests that expression of LMP1 alone is not responsible for the reduction of cIAP-1 in these cells. Bortezomib also reduced the expression of cIAP-2 and XIAP in LCLa cells (Fig. 7C). Bay 11-7082 also reduces the levels of cIAP-1, cIAP-2, and XIAP (Fig. 7C). In contrast, bortezomib did not affect the levels of Bcl-2, Bcl-XL, TRAF2, or LMP1 in LCLa cells (Fig. 7A). Similar results were obtained with LCL Bra and LCL Der cells (data not shown). Bortezomib did not affect the levels of cIAP-1, cIAP-2, and XIAP in Mutu I, EBV-positive Akata, and BJAB cells (data not shown).
FIG. 7.
Bortezomib down-regulates the expression of antiapoptotic proteins cIAP-1, cIAP-2, and XIAP. (A) LCLa cells were treated with or without 1 μM bortezomib (bort) for the indicated times, and whole-cell lysates were immunoblotted using antibodies to proteins involved in apoptosis. β-Actin was used as a protein loading control. (B) BJgpt-3, BJLMP6, BJLMP15, and LCLa cells were treated with (+) or without (−) 1 μM bortezomib for 6 h, and the lysates were immunoblotted with antibodies to LMP1, cIAP-1, or β-actin. (C) LCLa cells were treated with 1 μM bortezomib (bort) or 5 μM Bay 11-7082 (Bay); 6 h later, levels of cIAP-1, cIAP-2, and XIAP were determined by immunoblotting. β-Actin was used as a protein loading control.
Bortezomib increases survival of SCID mice inoculated with EBV-transformed B cells.
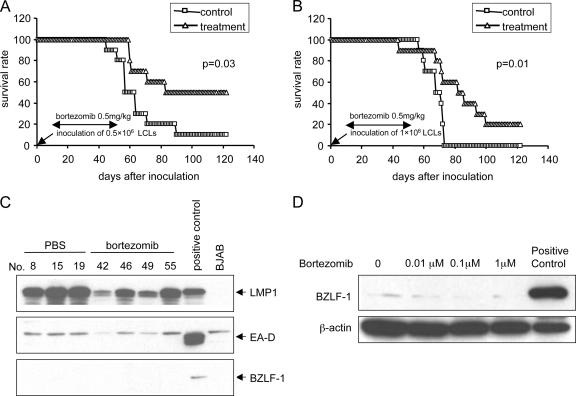
i.p. injection of SCID mice with EBV-transformed B cells results in development of EBV-positive lymphomas that express the same viral proteins as those present in tumors from immunosuppressed persons with EBV lymphoproliferative disease (42). Groups of 10 SCID mice were inoculated i.p. with LCLa cells (1 × 106 or 0.5 × 106), and 1 week later, mice were treated with bortezomib (0.5 mg/kg three times per week) or PBS i.p. for 6 weeks. Ten weeks after inoculation with 0.5 × 106 LCLs, 80% of the control mice were dead from lymphoma, while 60% of mice treated with bortezomib were still alive (Fig. 8A). Ten weeks after inoculation with 1.0 × 106 LCLs, 80% of the control mice were dead from lymphoma, while 60% of the mice treated with bortezomib were alive (Fig. 8B). Mice treated with bortezomib survived significantly longer than those receiving PBS (P = 0.03 for Fig. 8A, P = 0.01 for Fig. 8B). Similar results were obtained with mice inoculated with LCLb cells (data not shown). At autopsy, all of the control mice had immunoblastic lymphomas; all but two of the mice that died in the bortezomib group in each experiment had lymphomas. Immunoblot analysis of tumors from mice that received PBS or bortezomib showed expression of LMP1 protein but not the EBV immediate-early BZLF-1 protein or EA-D (Fig. 8C). Similarly, treatment of LCLs with bortezomib did not induce expression of the BZLF-1 protein in vitro (Fig. 8D).
FIG. 8.
Bortezomib increases survival of SCID mice inoculated with EBV-transformed B cells. Kaplan-Meier survival curves were plotted for SCID mice inoculated with 0.5 × 106 (A) or 1 × 106 (B) LCLa cells. Seven days after inoculation, mice were treated with bortezomib (triangles) or PBS (squares) for a total of 6 weeks. Bortezomib treatment was stopped at 6 weeks because of weight loss in the mice. (C) Tissue lysates from tumors of bortezomib-treated or control mice were used for immunoblotting with antibodies to EBV LMP1, BZLF-1 protein, or EA-D. Tissues were analyzed when mice were moribund, which was approximately 5 weeks after the last dose of bortezomib. (D) LCLa cells were treated with various concentrations of bortezomib for 24 h and immunoblotted with antibody to BZLF-1. P3HR-1 cells treated with phorbol myristate acetate and butyrate served as a positive control in panels C and D.
DISCUSSION
We have shown that bortezomib induces apoptosis of EBV-transformed LCLs in vitro and prolongs survival of SCID mice with EBV lymphomas. Bortezomib induced apoptosis of LCLs through both the caspase-8 and the caspase-9 pathways; pretreatment of the cells with the pan-caspase inhibitor Q-VD-OPH blocked the effect of bortezomib. LCLs were more sensitive to killing by bortezomib than Burkitt lymphoma cells or EBV-negative B cells. Mutu I and Mutu III are both Burkitt lymphoma cells derived from same lymphoma, but Mutu III cells express each of the EBV-associated latency genes, while Mutu I express only EBNA-1 (21). These findings suggest that bortezomib may interfere with an activity of one of the EBV latency gene products. Bortezomib at a dose of 1 μM resulted in over 70% loss of viability of LCLs and Mutu I and Mutu III cells (Fig. 1). Plasma levels in humans peak at 1.3 μM after a dose of 1.3 mg/m2 of bortezomib is received.
We found that bortezomib inhibited NF-κB DNA-binding activity within 2 hours of treatment with the drug. EBV LMP1 constitutively activates NF-κB in LCLs by both the canonical and the noncanonical pathways (4, 18, 32, 37). In the canonical pathway, the TES1 domain of LMP1 binds to TRAF1, TRAF2, TRAF3, and TRAF5 (7, 15, 16), while the TES2 domain of LMP1 binds to TRAF6, TRADD, and RIP (23). The complexes that are formed result in recruitment of the IκB kinases, which induce phosphorylation and degradation of IκBα, which subsequently allows the RelA and p50 subunits of NF-κB to translocate to the nucleus, where they activate transcription (32, 37). The proteasome processes NF-κB1 p105 into p50 in a translation-independent manner (35). Although we found an increase in the phosphorylation of IκBα in LCLs after exposure to bortezomib, we did not observe an increase in the total IκBα protein level (Fig. 4D). A similar increase in phosphorylation of IκBα was reported to occur in HTLV-1 transformed T cells treated with bortezomib (34, 46). One of these studies reported an increase (34), while another reported no change (46) in the level of total IκBα protein. These results suggest that bortezomib may not inhibit NF-κB solely though the canonical pathway.
LMP1 also activates the noncanonical pathway of NF-κB activation. In this pathway, the TES1 domain of LMP1 binds to TRAFs, which results in proteosome-mediated proteolysis of p100 to p52. Proteolysis of NF-κB2 p100 is regulated by the IKK1 (IKKα) subunit of the IKK complex (44). This results in translocation of p52 with the p65 and RelB subunits of NF-κB into the nucleus to activate transcription. Since bortezomib inhibited expression of p52 in LCLs, these results suggest that bortezomib may also inhibit the noncanonical pathway of NF-κB activation in LCLs. The noncanonical NF-κB pathway is known to be dysregulated in a number of malignancies (40, 49). Our observation that bortezomib was less effective in killing BJAB cells, which have low levels of constitutive NF-κB activity and whose survival depends on the expression of c-Myc and is independent of NF-κB (20), further supports the role of the NF-κB pathway in bortezomib-mediated cell death.
Bortezomib inhibited expression of three antiapoptotic proteins, cIAP-1, cIAP-2, and XIAP, in LCLs. These three IAP family members can directly inhibit caspases 3, 7, and 9 (14, 43). cIAP-1 and cIAP-2 expression is increased following activation of NF-κB (47). In contrast, bortezomib did not reduce levels of Bcl-2 or Bcl-XL. Bay 11-7082 and bortezomib both inhibited expression of IκBα in LCLs. LCLs treated with Bay 11-7082 showed decreased levels of transcripts for cIAP-2, cFLIP, and Bcl-XL (9, 26) and decreased protein levels of cIAP-1, cIAP-2, and cFLIP (26). These results indicate that bortezomib and Bay 11-7082 may induce apoptosis of LCLs through similar mechanisms of action.
We found that bortezomib was effective for treatment of EBV-associated lymphomas in SCID mice. Treatment of animals with bortezomib 1 week after injection with EBV-transformed B cells resulted in a statistically significant improvement in survival compared to what was found for control mice. Bortezomib has activity against a wide range of malignancies, including multiple myeloma, prostate cancer, breast cancer, colon cancer, and lung cancer (2, 30). NF-κB activation can maintain tumor cell survival, and inhibition of NF-κB alone is sufficient to induce apoptosis (8, 27). Bay 11-7082, which inhibits NF-κB activity, also inhibited the growth of EBV lymphomas in SCID mice (26); bortezomib, like Bay 11-7082, did not result in the induction of EBV lytic replication in SCID mice. These data suggest that bortezomib may have a role in the treatment of EBV lymphomas that occur in immunocompromised persons.
Acknowledgments
This study was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases.
We thank Fred Wang for BJgpt-3, BJLMP-6, and BJLMP-15 cells; Keith Brown for antibodies to p50 and p65; Jeffery Sample for Mutu cells; Yo Hoshino for assistance with flow cytometry; and Jin Qin for help with statistics. Bortezomib was provided by Millennium Pharmaceuticals, Inc., and the National Cancer Institute, NIH.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Adams, J. 2002. Proteasome inhibition: a novel approach to cancer therapy. Trends Mol. Med. 8:S49-S54. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. 2002. Proteasome inhibitors as new anticancer drugs. Curr. Opin. Oncol. 14:628-634. [DOI] [PubMed] [Google Scholar]
- 3.Aota, K., M. Azuma, T. Yamashita, T. Tamatani, K. Motegi, N. Ishimaru, Y. Hayashi, and M. Sato. 2000. 5-Fluorouracil induces apoptosis through the suppression of NF-kappaB activity in human salivary gland cancer cells. Biochem. Biophys. Res. Commun. 273:1168-1174. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson, P. G., H. J. Coope, M. Rowe, and S. C. Ley. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 278:51134-51142. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 6.Bours, V., G. Franzoso, V. Azarenko, S. Park, T. Kanno, K. Brown, and U. Siebenlist. 1993. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell 72:729-739. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur, S. R., G. Cheng, D. Baltimore, and D. A. Thorley-Lawson. 1997. Localization of the major NF-kappaB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J. Biol. Chem. 272:19777-19784. [DOI] [PubMed] [Google Scholar]
- 8.Cahir-McFarland, E., and E. Kieff. 2002. NF-kappaB inhibition in EBV-transformed lymphoblastoid cell lines. Recent Results Cancer Res. 159:44-48. [DOI] [PubMed] [Google Scholar]
- 9.Cahir-McFarland, E. D., K. Carter, A. Rosenwald, J. M. Giltnane, S. E. Henrickson, L. M. Staudt, and E. Kieff. 2004. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 78:4108-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 97:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caserta, T. M., A. N. Smith, A. D. Gultice, M. A. Reedy, and T. L. Brown. 2003. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8:345-352. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, J. I. 2000. Epstein-Barr virus infection. N. Engl. J. Med. 343:481-492. [DOI] [PubMed] [Google Scholar]
- 13.Cusack, J. C., Jr., R. Liu, M. Houston, K. Abendroth, P. J. Elliott, J. Adams, and A. S. Baldwin, Jr. 2001. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: Implications for systemic nuclear factor- kappaB inhibition. Cancer Res. 61:3535-3540. [PubMed] [Google Scholar]
- 14.Deveraux, Q. L., R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300-304. [DOI] [PubMed] [Google Scholar]
- 15.Devergne, O., E. D. Cahir-McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. F. Kleijnen, E. Kieff, and G. Mosialos. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol. 16:7098-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixit, V., and T. W. Mak. 2002. NF-kappaB signaling. Many roads lead to Madrid. Cell 111:615-619. [DOI] [PubMed] [Google Scholar]
- 18.Eliopoulos, A. G., J. H. Caamano, J. Flavell, G. M. Reynolds, P. G. Murray, J. L. Poyet, and L. S. Young. 2003. Epstein-Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-kappaB2 to p52, via an IKKgamma/NEMO-independent signaling pathway. Oncogene 22:7557-7569. [DOI] [PubMed] [Google Scholar]
- 19.Franzoso, G., P. Biswas, G. Poli, L. Carlson, K. D. Brown, M. Tomita-Yamaguchi, A. S. Fauci, and U. Siebenlist. 1994. A family of serine proteases expressed exclusively in myelo-monocytic cells specifically processes the nuclear factor-kappa B subunit p65 in vitro and may impair human immunodeficiency virus replication in these cells. J. Exp. Med. 180:1445-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glazer, P. M., and W. C. Summers. 1985. Oncogene expression in isogenic, EBV-positive and -negative Burkitt lymphoma cell lines. Intervirology 23:82-89. [DOI] [PubMed] [Google Scholar]
- 21.Gregory, C. D., M. Rowe, and A. B. Rickinson. 1990. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J. Gen. Virol. 71:1481-1495. [DOI] [PubMed] [Google Scholar]
- 22.Hideshima, T., P. Richardson, D. Chauhan, V. J. Palombella, P. J. Elliott, J. Adams, and K. C. Anderson. 2001. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 61:3071-3076. [PubMed] [Google Scholar]
- 23.Izumi, K. M., E. D. Cahir-McFarland, A. T. Ting, E. A. Riley, B. Seed, and E. D. Kieff. 1999. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-κB activation. Mol. Cell. Biol. 19:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson, G., H. Einsele, P. Moreau, J. S. Miguel, and J. S. Bortezomib. 2005. A novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treat. Rev. 31:591-602. [DOI] [PubMed] [Google Scholar]
- 25.Katano, H., L. Pesnicak, and J. I. Cohen. 2004. Simvastatin induces apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas. Proc. Natl. Acad. Sci. USA 101:4960-4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keller, S. A., D. Hernandez-Hopkins, J. Vider, V. Ponomarev, E. Hyjek, E. J. Schattner, and E. Cesarman. 2006. NF-kappaB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood 107:3295-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller, S. A., E. J. Schattner, and E. Cesarman. 2000. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96:2537-2542. [PubMed] [Google Scholar]
- 28.Kieff, E. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Kieff, E., and A. Rickinson. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 30.Lenz, H. J. 2003. Clinical update: proteasome inhibitors in solid tumors. Cancer Treat. Rev. 29(Suppl. 1):41-48. [DOI] [PubMed] [Google Scholar]
- 31.Liebowitz, D. 1998. Epstein-Barr virus and a cellular signaling pathway in lymphomas from immunosuppressed patients. N. Engl. J. Med. 338:1413-1421. [DOI] [PubMed] [Google Scholar]
- 32.Luftig, M., T. Yasui, V. Soni, M.-S. Kang, N. Jacobson, E. Cahir-McFarland, B. Seed, and E. Kieff. 2004. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc. Natl. Acad. Sci. USA 101:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menezes, J., W. Leibold, G. Klein, and G. Clements. 1975. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine 22:276-284. [PubMed] [Google Scholar]
- 34.Mitra-Kaushik, S., J. C. Harding, J. L. Hess, and L. Ratner. 2004. Effects of the proteasome inhibitor PS-341 on tumor growth in HTLV-1 Tax transgenic mice and Tax tumor transplants. Blood 104:802-809. [DOI] [PubMed] [Google Scholar]
- 35.Moorthy, A. K., O. V. Savinova, J. Q. Ho, V. Y. Wang, D. Vu, and G. Ghosh. 2006. The 20S proteasome processes NF-kappaB1 p105 into p50 in a translation-independent manner. EMBO J. 25:1945-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlowski, R. Z., J. R. Eswara, A. Lafond-Walker, M. R. Grever, M. Orlowski, and C. V. Dang. 1998. Tumor growth inhibition induced in a murine model of human Burkitt's lymphoma by a proteasome inhibitor. Cancer Res. 58:4342-4348. [PubMed] [Google Scholar]
- 37.Paine, E., R. I. Scheinman, A. S. Baldwin, Jr., and N. Raab-Traub. 1995. Expression of LMP1 in epithelial cells leads to the activation of a select subset of NF-κB/Rel family proteins. J. Virol. 69:4572-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 39.Ramalingam, P., W. S. Chu, R. Tubbs, L. Rybicki, J. Pettay, and E. D. Hsi. 2003. Latent membrane protein 1, tumor necrosis factor receptor-associated factor (TRAF) 1, TRAF-2, TRAF-3, and nuclear factor kappa B expression in posttransplantation lymphoproliferative disorders. Arch. Pathol. Lab. Med. 127:1335-1339. [DOI] [PubMed] [Google Scholar]
- 40.Rayet, B., and C. Gelinas. 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 41.Richardson, P. G., C. Mitsiades, T. Hideshima, and K. C. Anderson. 2006. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu. Rev. Med. 57:33-47. [DOI] [PubMed] [Google Scholar]
- 42.Rowe, M., L. S. Young, J. Crocker, H. Stokes, S. Henderson, and A. B. Rickinson. 1991. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J. Exp. Med. 173:147-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy, N., Q. L. Deveraux, R. Takahashi, G. S. Salvesen, and J. C. Reed. 1997. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16:6914-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 45.Takada, K., K. Horinouchi, Y. Ono, T. Aya, T. Osato, M. Takahashi, and S. Hayasaka. 1991. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147-156. [DOI] [PubMed] [Google Scholar]
- 46.Tan, C., and T. A. Waldmann. 2002. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 62:1083-1086. [PubMed] [Google Scholar]
- 47.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 48.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, X. M., H. Lin, C. Chen, and B. D. Chen. 1999. Inhibition of ubiquitin-proteasome pathway activates a caspase-3-like protease and induces Bcl-2 cleavage in human M-07e leukaemic cells. Biochem. J. 340:127-133. [PMC free article] [PubMed] [Google Scholar]