Abstract
The M RNA genome segment of Bunyamwera virus (BUNV), the prototype of the Bunyaviridae family, encodes a precursor polyprotein that is proteolytically cleaved to yield two structural proteins, Gn and Gc, and a nonstructural protein called NSm. Gn and Gc are type I integral transmembrane glycoproteins. The Gn protein contains a predicted cytoplasmic tail (CT) of 78 residues, and Gc has a shorter CT of 25 residues. Little is known about the role of the Gn and Gc CT domains in the virus replication cycle. We generated a series of mutant glycoprotein precursor constructs containing either deletions or alanine substitutions in the CT domains of Gn and Gc. We examined the effects of these mutations on glycoprotein maturation, cell surface expression, and low pH-induced syncytium formation. In addition, the effects of these mutations were also assessed using a reverse genetics-based virus assembly assay and a virus rescue system. Our results show that the CT domains of both Gn and Gc play crucial roles in BUNV-mediated membrane fusion, virus assembly, and morphogenesis.
There are more than 300 members in the family Bunyaviridae, which is divided into five genera, Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus. Bunyamwera virus (BUNV) is the prototype of both the family and the Orthobunyavirus genus (8, 34). Most bunyaviruses are arthropod-borne viruses, and several members are human pathogens, such as La Crosse virus (LACV), Hantaan virus, Rift Valley fever virus, and Crimean-Congo hemorrhagic fever virus (9). Bunyaviruses share similar morphological and biochemical characteristics, including possession of a tripartite, single-stranded, negative-sense RNA genome, a cytoplasmic site for viral replication and transcription, and maturation and budding at the Golgi complexes of infected cells (11, 38, 44).
Bunyavirus glycoproteins form projections or spikes on the viral envelope and are encoded by the medium segment (M) as a precursor polyprotein that is cotranslationally cleaved to yield the two mature proteins, called Gn and Gc. A nonstructural protein called NSm is also encoded in the M segments of orthobunyaviruses, some phleboviruses, and tospoviruses (8, 44). Both Gn and Gc are type I integral transmembrane proteins that are modified by N-linked glycosylation. The signal for Golgi retention and targeting of the BUNV glycoproteins was mapped to the transmembrane domain of the Gn protein, and heterodimerization between Gn and Gc is crucial for the Golgi transportation and maturation of the larger Gc protein (27, 47, 50). The BUNV Gn protein consists of 302 residues (Mr, 32,000) with a predicted cytoplasmic tail (CT) domain of 78 amino acids (residues 225 to 302). Gc is 953 residues in length (Mr, 110,000) and has a predicted shorter CT of just 25 amino acids (residues 1409 to 1433). An alignment of glycoprotein sequences from eight orthobunyaviruses shows that the Gn CT is highly conserved, showing 59% amino acid identity, in contrast to an overall 34% identity for the entire Gn protein. The Gc CT is less conserved, showing 32% amino acid identity, but this is still higher than the overall identity (27%) for whole Gc proteins (Fig. 1), suggesting that the CT domains may be important in virus replication.
FIG. 1.
BUNV M RNA segment and mutagenesis of the glycoprotein precursor. The layout of the BUNV M segment-encoded gene product is shown at the top, with positions of amino acid residues marking protein boundaries (Gn, NSm, and Gc) indicated. ss, signal peptide; TMD, transmembrane domain. Below are shown alignments of the CT domains of Gn and Gc proteins from eight orthobunyaviruses and sequences of the BUNV mutants containing sequential substitutions with strings of five alanine residues. Abbreviations: CEV, California encephalitis virus (GenBank database accession number AAD53039); LACV, La Crosse virus (AAB62804); INKV, Inkoo virus (AAB93841); SRV, South River virus (AAD53044); JCV, Jamestown Canyon virus (AAB93842); MAGV, Maguari virus (AAQ23639); NGAV, Ngari virus (AAT01931); BUNV, Bunyamwera virus (NP_047212).
Enveloped viruses assemble and bud by using different viral components and a range of strategies (for reviews, see references 13, 14, 33, 45, and 55). For some negative-strand RNA viruses, such as orthomyxoviruses, paramyxoviruses, and rhabdoviruses, the matrix protein, which forms a shell-like structure underneath the viral membrane, plays a crucial role in virus assembly and budding (13, 45). Since bunyaviruses do not contain a matrix protein, the CT domain of one or both of the two viral glycoproteins is presumed to be involved in the assembly and budding process, and it was recently reported for Uukuniemi phlebovirus that the CT domain of the Gn protein interacts with the viral ribonucleoprotein (RNP) during genome packaging (37). The CT domains of the glycoproteins of many other enveloped viruses have important roles in the viral life cycle, including glycoprotein transport and membrane insertion, virus-mediated cell fusion, and virus budding (5, 6, 21, 30, 36, 42, 54, 56, 60).
For this report, we constructed a series of glycoprotein precursor mutants, including two constructs with either the Gn CT or Gc CT deleted and 20 mutants with sequential alanine substitutions in the CT domains of the two glycoproteins. We investigated the role of the CT domains in virus assembly and morphogenesis with regard to glycoprotein maturation and cell surface expression, low pH-induced syncytium formation, production of infectious virus-like particles (VLPs), and virus viability. Our data showed that the CT domains of both Gn and Gc are highly sensitive to mutation and are indispensable for BUNV assembly and morphogenesis.
MATERIALS AND METHODS
Cells and viruses.
Vero E6 (ATCC C1008), BHK-21, and BSR-T7/5 cells (4) were maintained as described previously (47). Working stocks of wild-type (wt) and mutant BUNV were grown in BHK-21 cells, and titers were determined by plaque assay as detailed previously (57). A recombinant vaccinia virus that expresses T7 RNA polymerase, i.e., vTF7-3 (12), was provided by B. Moss (NIH, Bethesda, MD).
Antibodies.
A rabbit antiserum against purified BUNV virions (anti-BUNV) and a BUNV Gc-specific monoclonal antibody (MAb), MAb 742, have been described previously (27, 57). A rabbit polyclonal antibody against GM130, a cis-Golgi matrix protein (32), was provided by M. Lowe (School of Biological Sciences, University of Manchester, United Kingdom). A goat anti-rabbit antibody conjugated with fluorescein isothiocyanate was purchased from Sigma, and a goat anti-mouse antibody conjugated with Cy5 was purchased from Amersham Pharmacia Biotech (Buckingham, United Kingdom).
Plasmids.
Plasmids that either express BUNV proteins (pTM1-BUNL to express the L protein; pTM1-BUNM to express Gn, NSm, and Gc; and pTM1-BUNS to express N and NSs) or generate full-length antigenomic RNA transcripts [pT7riboBUNL(+), pT7riboBUNM(+), and pT7riboBUNS(+)] have been described previously (3, 28), as have the BUNV-derived minigenome pT7riboBUNMREN(−) (contains the Renilla luciferase gene) (58) and pTM1-FF-Luc, which contains the firefly luciferase gene in the vector pTM1 (31). The construct pTM1-BUNMΔGnCT (Gn224-Gc), which encodes the BUNV M segment open reading frame product with the Gn CT removed, has been described previously (50).
The construct pTM1-BUNMΔGcCT was derived from pTM1-BUNM and has the coding sequence for 25 residues of the Gc CT (residues 1409 to 1433) deleted (Fig. 1). Twenty other alanine substitution constructs were derived from TVT7R-BUNM, in which the BUNV M segment cDNA was cloned into TVT7R(0,0) (22; J. Steel and R. M. Elliott, unpublished data). Groups of five residues in the CT domain of the Gn or Gc protein were sequentially replaced by five alanine residues (Fig. 1). All mutated cDNAs were also cloned into pTM1 (31), using standard DNA cloning techniques, for use in the VLP assembly assay and for immunofluorescence staining. All constructs were confirmed by DNA sequence analysis. The primers used and details of PCR amplification are available upon request.
Indirect immunofluorescence staining.
Immunofluorescence assays were performed as previously described (48). Briefly, infected or transfected cells grown on glass coverslips with a 13-mm diameter were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). For observation of the intracellular viral glycoproteins and GM130, the fixed cells were permeabilized with 0.1% Triton X-100 in PBS before being stained with specific primary antibodies and secondary antibody conjugates. Samples for the detection of cell surface expression of viral proteins were not permeabilized. Localization of fluorescently labeled proteins was examined using either a Zeiss LSM confocal microscope or a Delta Vision 3.5 restoration microscope (Applied Precision, MA), as indicated in the figure legends.
Metabolic radiolabeling, immunoprecipitation, and endo H digestion.
Metabolic radiolabeling and immunoprecipitation of BUNV proteins were performed as described previously (47). Briefly, at 24 h posttransfection, cells were pulse labeled with 80 μCi [35S]methionine (Amersham Pharmacia Biotech) for 20 min and then chased for up to 90 min in the presence of excess unlabeled methionine. Cells were lysed on ice with 300 μl of nondenaturing radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 1% Triton X-100, 300 mM NaCl, 5 mM EDTA) containing a cocktail of protease inhibitors (Roche). BUNV glycoproteins were immunoprecipitated with either anti-BUNV or MAb 742 that had been conjugated to protein A-Sepharose beads (Sigma). The bound glycoproteins were denatured in 30 μl denaturing buffer (0.5% sodium dodecyl sulfate [SDS] and 1% β-mercaptoethanol) at 100°C for 10 min and then digested with 150 mU endoglycosidase H (endo H; New England Biolabs) for 20 h at 37°C in a 40-μl reaction mix containing 50 mM sodium citrate, pH 5.5, 0.5% SDS, and 1% β-mercaptoethanol. The treated samples were analyzed by SDS-12.5% polyacrylamide gel electrophoresis (SDS-12.5% PAGE) under reducing conditions.
Surface biotinylation of viral glycoproteins.
Cell surface proteins of BUNV-infected or BUNV M cDNA-transfected cells were biotinylated with EZ-Link sulfo-NHS-LC-LC-biotin (Pierce). Briefly, the cell monolayer was washed three times with ice-cold PBS (pH 8.0) and incubated with 1 ml of biotin reagent solution (0.5 mg/ml) for 30 min on ice. The cells were then washed with cold PBS and lysed in radioimmunoprecipitation assay buffer. The biotinylated BUNV glycoproteins were immunoprecipitated with anti-BUNV as described earlier, separated by SDS-12.5% PAGE under reducing conditions, and then probed with horseradish peroxidase-conjugated streptavidin (Amersham Pharmacia Biotech, Buckingham, United Kingdom). Signals were revealed by chemiluminescence (SuperSignal kit; Pierce).
BUNV glycoprotein fusion assay.
BSR-T7/5 cells grown in 12-well plates were transfected with 1.0 μg of either pTM1-BUNM or one of the mutated BUNV M cDNA constructs. At 24 h posttransfection, the medium was removed and replaced with low-pH medium (Hanks balanced salt solution buffered to pH 5.3). After 5 min, this was replaced by normal growth medium. Cell fusion was observed after incubation for 4 to 5 h at 37°C. Fusion was quantified by counting the numbers of cells and nuclei present in a microscopic field after fusion treatment. A fusion index (f) was calculated according to the equation f = [1 − (c/n)], where c is the number of cells in a field and n is the number of nuclei (59). An average field at a magnification of ×200 contained 300 to 400 nuclei. The average f for three fields was calculated.
BUNV assembly assay.
The assay for infectious VLP production was modified from a previously described method (49). Briefly, BSR-T7/5 cells were transfected with three expression constructs, pTM1-BUNS (0.05 μg), pTM1-BUNL (0.1 μg), and either pTM1-BUNM (0.1 μg) or one of the mutated M segment cDNAs cloned into pTM1 (also 0.1 μg), together with 0.1 μg of the BUNV-derived minigenome pT7riboBUNMREN(−) and 0.05 μg of pTM1-FF-Luc (internal transfection control). At 24 h posttransfection, the supernatant was used to infect new monolayers of BSR-T7/5 cells. Renilla luciferase activity was measured after 24 h of incubation, using a dual-luciferase assay kit (Promega) as described previously (24).
Virus rescue by reverse genetics.
Rescue experiments were performed as described previously (28). Briefly, BSR-T7/5 cells were transfected with a mixture of three plasmids, i.e., 1.5 μg each of pT7riboBUNL(+), pT7riboBUNS(+), and either TVT7R-BUNM(+) or one of the TVT7R-BUNM-derived M cDNA mutants. At 6 h posttransfection, cells were supplemented with 4 ml of growth medium and incubation was continued for 5 to 11 days, until a cytopathic effect was evident. The transfectant viruses were isolated by plaque formation on Vero E6 cells.
RESULTS
Requirement of the CT domains for maturation of the two viral glycoproteins.
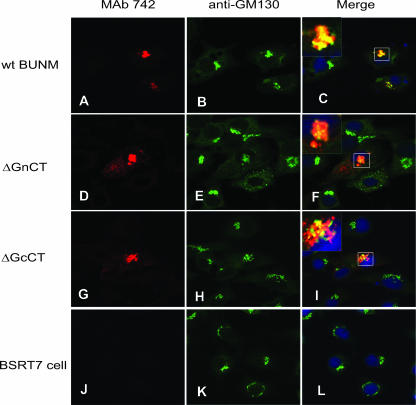
BUNV glycoproteins target to and accumulate in the Golgi complex, where they acquire endo H resistance (27, 50). These features were exploited to assess the effects of mutations in the CT domains on glycoprotein processing and localization. We previously reported that removal of the Gn CT affected Golgi localization and protein maturation (50); in the present study, the role of the Gc CT on Golgi targeting was addressed. As shown in Fig. 2, Gc expressed from full-length wt M segment cDNA colocalized with the cis-Golgi marker GM130 (panels A to C), while the tailless Gc protein expressed from the construct pTM1-BUNMΔGcCT partially colocalized with GM130 (panels G to I). Some Gc protein did not colocalize with GM130 but was still located in the Golgi region, while some Gc remained distributed in the cytoplasm. However, more Gc protein was observed in the cytoplasm when expressed from the construct pTM1-BUNMΔGnCT, in which the Gn CT was deleted (Fig. 2D to F). Together, these data suggest that the CT domains of both proteins play a role in the Golgi trafficking and maturation processes of BUNV glycoproteins.
FIG. 2.
Colocalization of Gc protein with Golgi matrix protein GM130. BSR-T7/5 cells were transfected with BUNV M segment cDNA constructs pTMBUNM (wt BUNM), BUNMΔGnCT (ΔGnCT), and BUNMΔGcCT (ΔGcCT) or were left untransfected (BSRT7 cell), as indicated. The permeabilized samples were stained with a mixture of anti-Gc MAb 742 and anti-GM130 and examined by confocal microscopy. Gc stains red, and the Golgi complex stains green. Colocalization is shown as yellow in the merged images, with an enlarged selected area shown in the upper left corner. The nuclei were stained blue by 4′,6′-diamidino-2-phenylindole (DAPI).
To examine further the role of the CT domains of both Gn and Gc in the protein folding process, cells were infected with the recombinant vaccinia virus vTF7-3, which expresses T7 RNA polymerase, and then transfected with either wt BUNV M cDNA or one of the mutant glycoprotein precursor constructs, i.e., pTM1-BUNMΔGnCT or pTM1-BUNMΔGcCT. Cells were pulse labeled for 20 min and then chased for up to 90 min in the presence of excess unlabeled methionine. (The vTF7-3 system was used in these experiments to achieve high levels of radioactive methionine incorporation with short labeling times.) Cell lysates were immunoprecipitated with either the conformation-specific anti-Gc MAb 742 or anti-BUNV, a polyclonal antiserum prepared against BUNV particles, and subjected to endo H treatment. As shown in Fig. 3A, MAb 742 precipitated Gc proteins from wt BUNV M, BUNMΔGnCT, and BUN MΔGcCT constructs, and these proteins were folded correctly and had acquired endo H resistance after 20 min of chase. Quantification of the protein bands by phosphorimagery revealed that peak levels of correctly folded Gc proteins expressed from the wt M construct and BUNMΔGcCT were seen after 40 min of chase, whereas the peak level of Gc from BUNMΔGnCT was achieved after 60 min of chase. In addition, Gc expressed from the BUNMΔGnCT construct exhibited reduced reactivity with MAb 742 during the chase period; the amount of Gc precipitated was about 40% that of its wt counterpart after 40 min of chase. The effect of the Gn CT domain on Gc protein maturation was confirmed by precipitation with anti-BUNV, which showed that the conversion of Gc from an endo H-sensitive to an endo H-resistant form was compromised by the deletion of the Gn CT domain (Fig. 3B). Nevertheless, deletion of the Gc CT domain, as shown by the pulse-chase experiments with both MAb 742 and anti-BUNV, had no negative effect on glycoprotein folding; instead, the tailless Gc protein seemed to be processed more efficiently than wt Gc. In all cases, the Gn protein was coimmunoprecipitated with Gc, indicating that the CT domains are not involved in Gn-Gc heterodimer formation.
FIG. 3.
Effect of deletion of either Gn or Gc CT on BUNV glycoprotein maturation. Vero cells were infected with vTF7-3 and subsequently transfected with wt or mutant BUNV M segment cDNA constructs. Cells were labeled with [35S]methionine for 20 min and chased in the presence of excess unlabeled methionine for different times, as indicated (in minutes). Labeled proteins were immunoprecipitated (IP) with either MAb 742 (A) or anti-BUNV (B), subjected to endo H digestion (+) or left undigested (−) as indicated, and analyzed by SDS-12.5% PAGE under reducing conditions. BUNV Gn and Gc proteins are indicated to the right of the gels. Quantitative analyses of protein bands by phosphorimaging are shown. Data from the pulse-chase experiment with MAb 742 are shown as the total density at each time point, while data from the pulse-chase experiment with anti-BUNV are shown as percentages of the endo H-resistant form versus the total Gc protein.
Mutations in CT domains affect cell surface expression of BUNV glycoproteins.
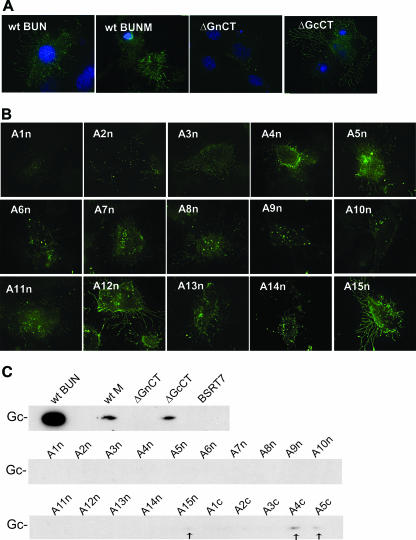
Detection of viral glycoproteins on the surfaces of infected cells has been reported for some bunyaviruses, such as hantaviruses (35, 41), LACV (20), and Uukuniemi virus (26). Here the role of the CT domains of both the Gn and Gc proteins in cell surface expression of BUNV glycoproteins was investigated by either immunofluorescence or a biotin-labeling technique. As shown in Fig. 4A, immunofluorescence staining of nonpermeabilized cells showed a dendriform or branch-like pattern of projections on either BUNV-infected or BUNV M segment cDNA-transfected cells (Fig. 4A, left two panels). Surface expression of the viral glycoproteins was confirmed by biotinylation, with the virus-infected cells giving a much stronger signal than those transfected with the M segment cDNA, reflecting the different efficiencies of infection and transfection (Fig. 4C). When the Gn CT was removed, no viral glycoprotein was detected by either immunofluorescence or surface biotinylation (Fig. 4A and C). However, removal of the Gc CT domain did not obviously affect the surface expression of the BUNV glycoproteins, with a staining pattern and expression level similar to those of the wt M control (Fig. 4A and C). It was noted that only the Gc protein was detected by surface biotinylation in both BUNV-infected and M segment cDNA-transfected cells.
FIG. 4.
Detection of BUNV glycoproteins on the cell surface. (A and B) Cell surface staining of nonpermeabilized cells. Cells were fixed with 4% paraformaldehyde and stained with anti-BUNV polyclonal antibody or anti-Gc MAb 742. Surface expression of viral glycoproteins was observed using a Delta Vision restoration microscope, and nuclei were stained blue with DAPI. (A) BSR-T7/5 cells were infected with wt BUNV or transfected with wt BUNV M segment cDNA (wt BUNM), BUNMΔGnCT (ΔGnCT), and BUNMΔGcCT (ΔGcCT) constructs, as indicated. Infected cells were stained with MAb 742, and the transfected cells were stained with anti-BUNV antibody. (B) BSR-T7/5 cells were transfected with BUNV M segment cDNA clones containing alanine substitutions in the Gn CT, as indicated. The cells were stained with anti-BUNV antibody. (C). Cell surface biotinylation of either wt BUNV-infected or alanine substitution mutant-transfected BSR-T7/5 cells. The biotinylated viral glycoproteins expressed on the cell surface were precipitated with anti-BUNV serum and separated in SDS-PAGE gels, and signals were revealed by chemiluminescence. No Gn protein was detected.
Analysis of the 15 alanine-scanning mutants revealed that alanine substitution in most regions of the Gn CT affected cell surface expression of the viral glycoproteins, and although cell surface expression was detectable, the pattern and density of surface staining were quite different from those of the wt control (Fig. 4B). The exceptions were constructs A5n, A12n, and A15n, which showed a wt staining pattern. No cell surface expression was observed for construct GnCTA1n, in which the five residues most proximal to the transmembrane domain were replaced by alanine residues. Surface biotinylation showed only a faint biotin signal of A15n (Fig. 4C, marked by an arrow), indicating that the mutations in the Gn CT region affected cell surface expression so severely that little, if any, biotinylated Gc was detectable. Taken together, our data indicate that the Gn CT domain plays a crucial role in the trafficking of viral glycoproteins to the cell surface.
Alanine substitution in the Gc CT did not obviously affect the surface immunofluorescence staining pattern of the viral glycoproteins (data not shown). However, surface biotinylation examination indicated that mutations in this region did reduce cell surface expression of the viral glycoproteins, with Gc expressed by A1c, A2c, and A3c being nearly undetectable and the signal for Gc expressed from A4c and A5c being reduced. These data suggest that the Gc CT may contain a motif that regulates the cell surface transport of the viral glycoproteins.
CT domains modulate low pH-induced syncytium formation.
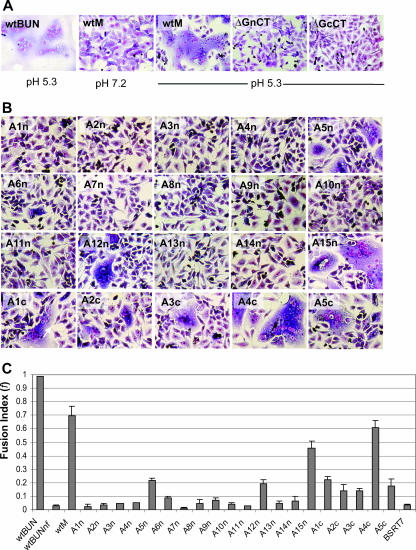
It has been noted that infection of cells with some bunyaviruses, such as the LACV and California encephalitis orthobunyaviruses (17, 18) or hantaviruses (2, 29), results in syncytium formation when the pH of the medium is lowered. We also observed extensive syncytia in cells infected with BUNV after low-pH (pH 5.3) treatment (Fig. 5A, first panel). Low pH-induced cell fusion was also shown in BSR-T7/5 cells that had been transfected with the full-length M segment cDNA construct (Fig. 5A, compare second and third panels). However, removal of the CT domain from either Gn or Gc led to the abrogation of syncytium formation (Fig. 3A, last two panels).
FIG. 5.
Low pH-induced syncytium formation. BSR-T7/5 cells were infected with BUNV or transfected with wt or mutant BUNV M segment cDNA constructs. At 24 h postinfection or -transfection, cells were treated with low-pH medium (pH 5.3) for 5 min, and syncytium formation was examined following incubation at 37°C for a further 5 h. Cells were then stained with Giemsa solution. (A) Syncytium formation in cells either infected with BUNV (wtBUN) or transfected with wt BUNV M segment cDNA (wtM), BUNMΔGnCT (ΔGnCT), and BUNMΔGcCT (ΔGcCT) constructs, as indicated. Cells were treated with acidic pH (pH 5.3) or neutral pH (pH 7.2) as shown below. (B) Syncytium formation following low-pH treatment in cells transfected with BUNV M segment mutants containing alanine substitutions in the Gn and Gc CTs. (C) f values. All f values were calculated from cells treated with low-pH (5.3) medium as described in Materials and Methods, except for the wtBUNnf value (second bar), which was obtained from wt BUNV-infected cells under neutral-pH conditions.
To understand further the role of the CT domains in membrane fusion, we examined the effects of mutations in either the Gn or Gc CT domain. Analysis of 15 alanine substitution mutants in the Gn CT revealed that the majority of the residues in the domain are crucial for fusogenic activity. Mutants A1n to A4n (involving residues 226 to 245), A6n to A11n (residues 251 to 280), and A13n and A14n (residues 286 to 295) resulted in abrogation of syncytium formation (Fig. 5B), with f values below 0.1 (Fig. 5C). Cell fusion was observed in cells transfected with three Gn CT mutants, A5n, A12n, and A15n, with f values of 0.216, 0.192, and 0.459, respectively (Fig. 5C). From these results, it seems that most residues in the Gn CT domain are crucial for the fusogenic function.
Although all five alanine-substituted mutant constructs of the Gc CT (A1c to A5c) mediated syncytium formation (Fig. 5B), mutation of the majority of the residues in the Gc CT (residues 1409 to 1423 and 1429 to 1433) reduced fusogenic activity; only construct A4c produce syncytia comparable to those of the wt control (Fig. 5B and C).
Requirement of Gn and Gc CT domains for virus assembly.
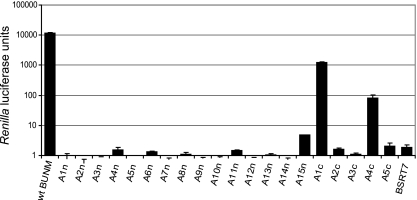
We described previously an assembly assay to monitor the formation of infectious VLPs that package a BUNV minigenome (49). Packaging, as a surrogate for assembly, was measured by the transfer of reporter gene activity (encoded by the minigenome) into cells “infected” with the VLPs. Using this assay, we showed that deletion of the entire CT domain of either Gn or Gc abolished the production of infectious VLPs; similarly, no VLPs were produced from constructs with internal deletions in the Gn CT (data not shown). When the alanine-scanning mutants were used in the assay, substitutions in any region of the Gn CT domain led to either complete abolition (A1n to A14n) or marked reduction (A15n) in packaging of the minigenome (Fig. 6). For A15n, the clone with the highest packaging signal of any of the Gn CT mutants, the activity was only 0.00038% that of the wt M segment cDNA control activity.
FIG. 6.
Effects of mutations in CT domains of Gn and Gc on the formation of infectious VLPs. BSR-T7/5 cells were transfected with minigenome component plasmids together with either wt BUNV M segment cDNA or alanine-substituted mutants. Supernatants from these cells were taken at 24 h posttransfection and used to infect fresh BSR-T7/5 cells. Renilla luciferase activities in extracts of these cells were measured after another 24 h of incubation and are shown in arbitrary light units.
Alanine substitution in the Gc CT domain had relatively less impact on virus assembly. Substitution at two regions, residues 1409 to 1413 (construct A1c) and residues 1424 to 1428 (A4c), showed 10.3% and 0.068% activity compared to that of the wt. However, substitutions in other regions, namely, residues 1414 to 1423 (A2c and A3c) and residues 1429 to 1433 (A5c), also abolished VLP assembly.
Effects of mutations in CT domains on virus viability.
The role of the CT domains in virus replication was assessed further by using our efficient BUNV reverse genetics rescue system (28). In agreement with the observations described above, rescue experiments showed that most of the mutations in the Gn and Gc CT domains, including removal of the complete CT domains (BUNMΔGnCT and BUNMΔGcCT), alanine substitutions in the Gn CT (A1n to A15n), and three alanine substitutions in the Gc CT (A2c, A3c, and A5c), were lethal to virus replication. However, we did manage to rescue two recombinant viruses, those with constructs GcCTA1c and GcCTA4c (designed rBUNGcCTA1 and rBUNGcCTA4, respectively). This correlated with our earlier finding that constructs A1c and A4c gave significant luciferase signals in the virus assembly assay. The recombinant viruses had a small-plaque phenotype and grew to low titers (>100-fold lower than that of the wt), indicating a degree of attenuation (Fig. 7). It was notable that the plaques formed by rGcCTA1 on Vero cells were too small to be seen by the naked eye. The virus rescue experiment further confirmed that both the Gn and Gc CT domains, and in particular the Gn CT, play a crucial role in virus replication.
FIG. 7.
Rescue of mutant viruses. The rescue of viruses containing deletions or alanine substitutions in the CT domains was attempted as described in Materials and Methods. Recombinant viruses were obtained from transfections involving BUNMGcCTA1 and BUNMGcA4 DNAs. (A) Yields of transfectant viruses rBUNGcCTA1 and rBUNGcA4. Supernatants from the initial transfection dishes were titrated by plaque assay on Vero cells. The results are the averages for two independent titrations. (B) Comparison of plaque sizes produced on Vero cells. The right column represents typical images of single plaques under a light microscope (magnification, ×100). Cell monolayers were fixed with 4% formaldehyde-PBS and stained with Giemsa solution.
DISCUSSION
Bunyaviruses enter cells by receptor-mediated endocytosis, and they mature and bud in the Golgi complex due to the accumulation of the two glycoproteins (Gn and Gc) in this organelle (25, 38, 43, 44). Unlike most other negative-strand RNA viruses, bunyaviruses do not carry a matrix protein, and it was presumed that the CT domains of Gn and Gc proteins play a crucial role in the virus assembly process. Indeed, recent data from Overby et al. (37) showed that the Gn CT of Uukuniemi phlebovirus is essential for genome packaging, and our work presented herein confirms that the CT domains of both Gn and Gc are indispensable for BUNV replication with regard to their roles in protein maturation, cell surface expression of the glycoproteins, low pH-induced cell fusion, and virus assembly and viability.
We previously noted that removal of the Gn CT from the BUNV glycoprotein precursor impaired the retention and maturation of Gc in the Golgi complex (50). In this work, we found that deletion of the Gc CT also affected Golgi targeting and retention of Gc in transfected cells. The tailless Gc protein was shown to partially colocalize with the Golgi marker GM130. Pulse-chase analysis with either MAb 742, a conformation-sensitive antibody against Gc, or anti-BUNV antiserum revealed that deletion of either the Gn or Gc CT domain did not block the heterodimerization and maturation of the viral glycoproteins. The Gc proteins expressed by the two CT deletion mutants (BUNMΔGnCT and BUNMΔGcCT) acquired endo H resistance within 20 min. However, we noticed that removal of the Gn CT compromised the efficiency of Gc maturation, as monitored by the acquisition of endo H resistance, with a reduction in Gc immunoreactivity with MAb 742 and a delay in the time taken for correct folding of Gc. This was not surprising, as we demonstrated before that correct folding of Gc depends on the chaperone-like activity of Gn (47).
Surface detection of viral glycoproteins has been reported late in infection for some hantaviruses and Uukuniemi phlebovirus (35, 38, 41). As described herein, we detected glycoproteins directly on the surfaces of virus-infected and M segment cDNA-transfected cells, where dendriform or branch-like projections were observed. The cause of these morphological changes to the cell surface is not known, but they might represent sites of release of virion particles or VLPs or alteration of the cell surface due to viral glycoprotein accumulation in the membrane. Further work, especially observation with more sensitive microscopic methods, is needed to address this issue. In cells infected with New World hantavirus, tubular projections have also been observed at the cell surface; the projections could be stained immunologically for the viral glycoproteins, and occasionally virus particles were seen associated with the tubules (16, 53). In addition, virus budding at the plasma membrane was observed in cells infected with Sin Nombre and Black Creek Canal hantaviruses (16, 41) and in hepatocytes infected with Rift Valley fever phlebovirus (1).
Gn and Gc form a heterodimer in both BUNV-infected and M segment cDNA-transfected cells (27, 50). Since only the Gc protein was detected by using surface biotinylation, we presume that the smaller Gn protein was masked by its larger counterpart. This situation is reminiscent of that of the E1 glycoprotein of Sindbis virus, in which the E1 protein is buried almost completely in the virus structure (46).
Fusion of the virion envelope with the membrane of a host cell is a critical event that delivers the viral genome into the cytoplasm to initiate the virus replication cycle (19, 51, 52). For many viruses, including bunyaviruses, the mildly acidic milieu within the late endosomes is required for the fusion process (7). In addition, many viruses, including several bunyaviruses, have been reported to mediate syncytium formation (fusion from within) under low-pH conditions (2, 17, 20, 29). Computational analyses predicted the Gc glycoproteins of bunyaviruses to be low-pH-dependent class II fusion proteins (15). Recent work by Plassmeyer and colleagues provided experimental evidence that supports a role for LACV Gc as the fusion peptide (39, 40). They showed that mutation of the predicted fusion domain, residues 1066 to 1087, affected protein expression, shifted the pH threshold for fusion, and reduced or abrogated overall cell fusion. This domain is highly conserved between LACV and BUNV Gc proteins (10).
In this paper, we demonstrate that both Gn and Gc CT domains are also required for fusogenic activity, as evidenced by the observation that low pH-induced syncytium formation was affected or even abolished by mutations in or removal of the Gn or Gc CT. These findings suggest that the CT domains of both glycoproteins are involved in the fusion process, which requires the transition of the fusion protein from the metastable conformation to the fusion state (7, 19, 23).
Our efficient BUNV rescue system and the VLP assembly assay (28, 49) enabled us to evaluate the role of Gn and Gc CT domains in virus assembly and infectivity. Our data corroborated the above findings concerning the impact of mutations in the Gn and Gc CT domains on low pH-induced cell fusion and surface expression of the viral glycoproteins. All mutations in the Gn CT abrogated the production of infectious VLPs, and consequently, no viable viruses were recovered. Infectious VLP formation and subsequent recovery of recombinant viruses were achieved with only two mutant glycoproteins, namely, A1c (where residues 409 to 413 were replaced with alanine residues) and A4c (where residues 424 to 428 were replaced). The transfectant viruses, rBUNMGcCTA1 and rBUNMGcCTA4, were recovered at low yields, grew poorly, and had a small plaque size, indicating that the two mutant viruses were considerably attenuated.
Taken together, our data indicate that the CT domains of Gn and Gc play crucial roles in BUNV replication. We postulate that the CT domains of BUNV glycoproteins are involved in multiple events in virus entry and morphogenesis. After the entry of the virion particles by receptor-mediated endocytosis, the low pH of late endosomes triggers a conformational change of the viral glycoproteins to promote the transition from the metastable to the fusion state to initiate the fusion process. In the Golgi complex, the interaction of the Gn CT with the viral RNPs instigates virus assembly. The assembled virion particles either bud in the Golgi complex or are transported to the cytoplasmic membrane for release.
Acknowledgments
We thank Klaus Conzelmann, Martin Lowe, and Bernard Moss for the provision of reagents used in this study and Steve Lodmell for helpful comments on the manuscript.
This study was supported by grants from the Biotechnology and Biological Sciences Research Council and the Wellcome Trust to R.M.E.
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Anderson, G. W., and J. F. Smith. 1987. Immunoelectron microscopy of Rift Valley fever viral morphogenesis in primary rat hepatocytes. Virology 161:91-100. [DOI] [PubMed] [Google Scholar]
- 2.Arikawa, J., I. Takashima, and N. Hashimoto. 1985. Cell fusion by haemorrhagic fever with renal syndrome (HFRS) viruses and its application for titration of virus infectivity and neutralizing antibody. Arch. Virol. 86:303-313. [DOI] [PubMed] [Google Scholar]
- 3.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93:15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celma, C. C. P., J. M. Manrique, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Domains in the simian immunodeficiency virus gp41 cytoplasmic tail required for envelope incorporation into particles. Virology 283:253-261. [DOI] [PubMed] [Google Scholar]
- 6.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, R., M. Bouloy, C. H. Calisher, R. Goldbach, J. T. Moyer, S. T. Nichol, R. Pettersson, A. Plyusnin, and C. S. Schmaljohn. 2000. Bunyaviridae, p. 599-621. In M. H. V. van Regenmortel, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA.
- 9.Elliott, R. M. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3:572-577. [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott, R. M. 1990. Molecular biology of the Bunyaviridae. J. Gen. Virol. 71:501-522. [DOI] [PubMed] [Google Scholar]
- 11.Elliott, R. M. (ed.). 1996. The Bunyaviridae. Plenum Press, Inc., New York, NY.
- 12.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garoff, H., R. Hewson, and D. J. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garoff, H., M. Sjoberg, and R. H. Cheng. 2004. Budding of alphaviruses. Virus Res. 106:103-116. [DOI] [PubMed] [Google Scholar]
- 15.Garry, C. E., and R. F. Garry. 2004. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of bunyaviruses are class II viral fusion protein (beta-penetrenes). Theor. Biol. Med. Model. 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith, C. S., L. H. Elliott, C. J. Peters, and S. R. Zaki. 1995. Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch. Virol. 140:2107-2122. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Scarano, F., N. Pobjecky, and N. Nathanson. 1984. La Crosse bunyavirus can mediate pH-dependent fusion from without. Virology 132:222-225. [DOI] [PubMed] [Google Scholar]
- 18.Hacker, J. K., and J. L. Hardy. 1997. Adsorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: a role for G1. Virology 235:40-47. [DOI] [PubMed] [Google Scholar]
- 19.Harrison, S. C. 2005. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 64:231-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacoby, D. R., C. Cooke, I. Prabakaran, J. Boland, N. Nathanson, and F. Gonzalez-Scarano. 1993. Expression of the La Crosse M segment proteins in a recombinant vaccinia expression system mediates pH-dependent cellular fusion. Virology 193:993-996. [DOI] [PubMed] [Google Scholar]
- 21.Jin, H., G. P. Leser, J. Zhang, and R. A. Lamb. 1997. Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J. 16:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, K. N., J.-L. Zeddam, and L. A. Ball. 2000. Characterization and construction of functional cDNA clones of Pariacoto virus, the first alphanodavirus isolated outside Australasia. J. Virol. 74:5123-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kielian, M. 2006. Class II virus membrane fusion proteins. Virology 344:38-47. [DOI] [PubMed] [Google Scholar]
- 24.Kohl, A., T. J. Hart, C. Noonan, E. Royall, L. O. Roberts, and R. M. Elliott. 2004. A Bunyamwera virus minireplicon system in mosquito cells. J. Virol. 78:5679-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuismanen, E., K. Hedman, J. Saraste, and R. F. Pettersson. 1982. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol. Cell. Biol. 2:1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuismanen, E., J. Saraste, and R. F. Pettersson. 1985. Effect of monensin on the assembly of Uukuniemi virus in the Golgi complex. J. Virol. 55:813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lappin, D. F., G. W. Nakitare, J. W. Palfreyman, and R. M. Elliott. 1994. Localization of Bunyamwera bunyavirus G1 glycoprotein to the Golgi requires association with G2 but not with NSm. J. Gen. Virol. 75:3441-3451. [DOI] [PubMed] [Google Scholar]
- 28.Lowen, A. C., C. Noonan, A. McLees, and R. M. Elliott. 2004. Efficient bunyavirus rescue from cloned cDNA. Virology 330:493-500. [DOI] [PubMed] [Google Scholar]
- 29.McCaughey, C., X. Shi, R. M. Elliot, D. E. Wyatt, H. J. O'Neill, and P. V. Coyle. 1999. Low pH-induced cytopathic effect—a survey of seven hantavirus strains. J. Virol. Methods 81:193-197. [DOI] [PubMed] [Google Scholar]
- 30.Moll, M., H. D. Klenk, and A. Maisner. 2002. Importance of the cytoplasmic tails of the measles virus glycoproteins for fusogenic activity and the generation of recombinant measles viruses. J. Virol. 76:7174-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. Product review. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, N., C. Rabouille, R. Watson, T. Nilsson, N. Hui, P. Slusarewicz, T. E. Kreis, and G. Warren. 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131:1715-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayak, D. P., E. K.-W. Hui, and S. Barman. 2004. Assembly and budding of influenza virus. Virus Res. 106:147-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichol, S. T., B. Beaty, R. M. Elliott, R. Goldbach, A. Plyusnin, A. L. Schmaljohn, and R. B. Tesh. 2005. Bunyaviridae, p. 695-716. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Elsevier, Amsterdam, The Netherlands.
- 35.Ogino, M., K. Yoshimatsu, H. Ebihara, K. Araki, B.-H. Lee, M. Okumura, and J. Arikawa. 2004. Cell fusion activities of Hantaan virus envelope glycoproteins. J. Virol. 78:10776-10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oomens, A. G., K. P. Bevis, and G. W. Wertz. 2006. The cytoplasmic tail of the human respiratory syncytial virus F protein plays critical roles in cellular localization of the F protein and infectious progeny production. J. Virol. 80:10465-10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overby, A. K., R. F. Pettersson, and E. P. A. Neve. 2007. The glycoprotein cytoplasmic tail of Uukuniemi virus (Bunyaviridae) interacts with ribonucleoproteins and is critical for genome packaging. J. Virol. 81:3198-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersson, R. F., and L. Melin. 1996. Synthesis, assembly, and intracellular transport of Bunyaviridae membrane proteins, p. 159-188. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, NY.
- 39.Plassmeyer, M. L., S. S. Soldan, K. M. Stachelek, J. Martin-Garcia, and F. Gonzalez-Scarano. 2005. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 338:121-132. [DOI] [PubMed] [Google Scholar]
- 40.Plassmeyer, M. L., S. S. Soldan, K. M. Stachelek, S. M. Roth, J. Martin-Garcia, and F. Gonzalez-Scarano. 2007. Mutagenesis of the La Crosse virus glycoprotein supports a role for Gc (1066-1087) as the fusion peptide. Virology 358:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravkov, E., S. Nichol, and R. Compans. 1997. Polarized entry and release in epithelial cells of Black Creek Canal virus, a New World hantavirus. J. Virol. 71:1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruel, N., A. Zago, and P. G. Spear. 2006. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology 346:229-237. [DOI] [PubMed] [Google Scholar]
- 43.Salanueva, I. J., R. R. Novoa, P. Cabezas, C. Lopez-Iglesias, J. L. Carrascosa, R. M. Elliott, and C. Risco. 2003. Polymorphism and structural maturation of Bunyamwera virus in Golgi and post-Golgi compartments. J. Virol. 77:1368-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmaljohn, C., and J. W. Hooper. 2001. Bunyaviridae: the viruses and their replication, p. 1581-1602. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 45.Schmitt, A. P., and R. A. Lamb. 2004. Escaping from the cell: assembly and budding of negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:145-196. [DOI] [PubMed] [Google Scholar]
- 46.Sharp, J. S., S. Nelson, D. Brown, and K. B. Tomer. 2006. Structural characterization of the E2 glycoprotein from Sindbis by lysine biotinylation and LC-MS/MS. Virology 348:216-223. [DOI] [PubMed] [Google Scholar]
- 47.Shi, X., K. Brauburger, and R. M. Elliott. 2005. Role of N-linked glycans on Bunyamwera virus glycoproteins in intracellular trafficking, protein folding, and virus infectivity. J. Virol. 79:13725-13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi, X., and R. M. Elliott. 2002. Golgi localization of Hantaan virus glycoproteins requires coexpression of G1 and G2. Virology 300:31-38. [DOI] [PubMed] [Google Scholar]
- 49.Shi, X., A. Kohl, V. Léonard, P. Li, A. McLees, and R. Elliott. 2006. Requirement of the N-terminal region of the orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J. Virol. 80:8089-8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi, X., D. F. Lappin, and R. M. Elliott. 2004. Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins. J. Virol. 78:10793-10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieczkarski, S. B., and G. R. Whittaker. 2005. Viral entry. Curr. Top. Microbiol. Immunol. 285:1-23. [DOI] [PubMed] [Google Scholar]
- 52.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 53.Spiropoulou, C. F. 2001. Hantavirus maturation. Curr. Top. Microbiol. Immunol. 256:33-46. [DOI] [PubMed] [Google Scholar]
- 54.Takimoto, T., T. Bousse, E. C. Coronel, R. A. Scroggs, and A. Portner. 1998. Cytoplasmic domain of Sendai virus HN protein contains a specific sequence required for its incorporation into virions. J. Virol. 72:9747-9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takimoto, T., and A. Portner. 2004. Molecular mechanism of paramyxovirus budding. Virus Res. 106:133-145. [DOI] [PubMed] [Google Scholar]
- 56.Waning, D. L., A. P. Schmitt, G. P. Leser, and R. A. Lamb. 2002. Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5. J. Virol. 76:9284-9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watret, G. E., C. R. Pringle, and R. M. Elliott. 1985. Synthesis of bunyavirus-specific proteins in a continuous cell line (XTC-2) derived from Xenopus laevis. J. Gen. Virol. 66:473-482. [DOI] [PubMed] [Google Scholar]
- 58.Weber, F., E. F. Dunn, A. Bridgen, and R. M. Elliott. 2001. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology 281:67-74. [DOI] [PubMed] [Google Scholar]
- 59.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youn, S., E. W. Collisson, and C. E. Machamer. 2005. Contribution of trafficking signals in the cytoplasmic tail of the infectious bronchitis virus spike protein to virus infection. J. Virol. 79:13209-13217. [DOI] [PMC free article] [PubMed] [Google Scholar]