Abstract
Maintenance of Kaposi's sarcoma-associated herpesvirus (KSHV) episomes in latently infected cells is dependent on the latency-associated nuclear antigen (LANA). LANA binds to the viral terminal repeats (TR), leading to recruitment of cellular origin recognition complex proteins. Additionally, LANA tethers episomes to chromosomes via interactions with histones H2A and H2B (A. J. Barbera et al., Science 311:856-861, 2006). Despite these molecular details, less is known about how episomes are established after de novo infection. To address this, we measured short-term retention rates of green fluorescent protein-expressing replicons in proliferating lymphoid cells. In the absence of antibiotic selection, LANA significantly reduced the loss rate of TR-containing replicons. Additionally, we found that LANA can support long-term stability of KSHV replicons for more than 2 months under nonselective conditions. Analysis of cis elements within TR that confer episome replication and partitioning revealed that these activities can occur independently, and furthermore, both events contribute to episome stability. We found that replication-deficient plasmids containing LANA binding sites (LBS1/2) exhibited measurable retention rates in the presence of LANA. To confirm these observations, we uncoupled KSHV replication and partitioning by constructing hybrid origins containing the Epstein-Barr virus (EBV) dyad symmetry for plasmid replication and KSHV LBS1/2. We demonstrate that multiple LBS1/2 function in a manner analogous to that of the EBV family of repeats by forming an array of LANA binding sites for partitioning of KSHV genomes. Our data suggest that the efficiency with which KSHV establishes latency is dependent on multiple LANA activities, which stabilize viral genomes early after de novo infection.
Kaposi's sarcoma-associated herpesvirus (KSHV/human herpesvirus 8) is a DNA tumor virus present in Kaposi's sarcoma (KS) and lymphoproliferative diseases, such as primary effusion lymphoma (PEL) and multicentric Castleman's disease. As with other DNA tumor viruses, including Epstein-Barr virus (EBV) and papillomaviruses, KSHV genomes are maintained as multicopy episomes in the nuclei of latently infected cells (13, 49).
Conceptually, maintenance of viral episomes in dividing cells can be described as the sum of two distinct processes: (i) DNA replication and (ii) partitioning/segregation. Critical for episome maintenance are virally encoded origin binding proteins (OBPs), which support DNA replication by binding to cis-regulatory elements within their respective origins of replication. The latency-associated nuclear antigen (LANA) of KSHV is a functional homologue of the EBV nuclear antigen 1 (EBNA-1) in that it is the only viral protein required for episome maintenance (5, 16, 72). LANA binds cooperatively to two LANA binding sites (LBS1/2) (20) within the 801-bp highly G+C-rich terminal repeats (TR), 35 to 45 copies of which flank the unique long coding region of KSHV (36). LANA interacts with the cellular origin recognition complex, which assembles at TR in late G1/early S phase, thus eliciting replication (42, 65, 66). Our laboratory identified a 32-bp replication element (RE) directly adjacent to LBS1/2 within the TR that is absolutely required for LANA-dependent replication (29), and plasmids containing the minimal replicator (RE and LBS1/2) replicate in synchrony with host chromosomes once per cell cycle (67). While the minimal cis-regulatory elements for replication have been defined (29), whether additional cis elements within TR and/or the number of LANA binding sites within TR have a direct role in episome partitioning and maintenance has not been determined.
The first evidence demonstrating that LANA plays a key role in partitioning of viral episomes came from experiments involving G418 selection of Z6 cosmids harboring multiple TR copies (5). Subsequently it was shown that under selection, two copies of TR are required to efficiently maintain plasmids in a LANA-dependent fashion while one copy of TR conveys maintenance with less efficiency (6). Hence, all necessary cis-regulatory elements for both initiation of latent DNA replication and episome partitioning are located within TR sequences.
Extensive studies have been done on EBV oriP, a 1.8-kbp-long region containing two distinct cis elements: the dyad symmetry (DS) and the family of repeats (FR) (43, 71). EBNA-1 recruits the origin recognition complex to oriP (15) and facilitates long-term maintenance of oriP plasmids (72). The DS contains four EBNA-1 binding sites and functions as a replication origin, while the FR contains multiple EBNA-1 binding sites to facilitate episome partitioning (25, 51, 52). The organization of cis elements within the latent replication origins of EBV and KSHV exhibits some similarities in that the spacing between OBP binding sites is 21 bp for EBNA-1 compared to 22 bp for LANA (29). Unlike EBV, however, KSHV genomes do not contain an obvious FR element. Given that KSHV genomes have 35 to 45 TR copies, each containing high-affinity LANA binding sites, we hypothesized that multiple LBS1/2 function as a cis-partitioning element in a manner analogous to that of FR.
LANA, encoded by ORF73, is 222 to 234 kDa in size and can be divided into distinct functional domains. Piolot et al. first demonstrated that amino acids 5 to 22 within the proline-rich N terminus of LANA are required for the tethering of viral episomes to mitotic chromosomes (48). Furthermore, this N-terminal domain conveys chromosomal attachment when fused to heterologous proteins, such as green fluorescent protein (GFP) (34, 48). Consistent with the tethering model, LANA converges at sites along metaphase chromosomes in the presence of TR DNA (16). Several LANA-interacting chromatin-associated proteins, including Brd4, Brd2/RING3, HP1α, histone methyltransferase SUV39H1, methyl CpG binding protein MeCP2, and Dek, have been proposed as potential targets for LANA-dependent episome tethering (34, 41, 46, 55, 69). Recently Barbera et al. provided biochemical and genetic evidence that the LANA N terminus interacts directly with core histones H2A and H2B for episome tethering (8). The LANA C terminus contains a sequence-specific DNA binding domain (DBD) and a dimerization domain, both of which are required for DNA replication (20, 32, 42, 57). As such, the N terminus of LANA is responsible for tethering viral episomes that are bound by the C terminus to host chromosomes.
Both the N and C termini of LANA can facilitate multiple protein-protein interactions with cellular proteins, including members of the wnt family of transcriptional regulators and the tumor suppressor proteins RB and p53, although recently it has been shown that p53 pathways in PEL cells are intact (18, 19, 47, 50). As a result, LANA modulates both cellular and viral gene expression (44, 53).
In contrast to the growing knowledge on the molecular details by which LANA contributes to the initiation of DNA replication and tethering of episomes, a lot less is known about how episome maintenance is established. Indeed, studies of either ex vivo-cultivated KS tumor cells or de novo-infected cells suggest that these processes are rather inefficient (1, 3, 9, 14, 35). While PEL-derived cell lines can be readily established and contain 20 to 150 stable copies, KS-derived endothelial cells rapidly lose viral genomes upon ex vivo cultivation. Additionally, in vitro infection studies have revealed that although many cell types are susceptible to infection with KSHV, cells fail to establish stable latency and lose viral genomes (9, 35). Similar observations have been made with both KSHV and EBV replicons; in the absence of antibiotic selection, rapid loss of TR-containing or oriP plasmids from transfected cells has been reported, and provision of LANA or EBNA-1 had no measurable effect (22, 39).
Based on these observations, we and others have hypothesized that the establishment of latency, defined here as stable episomal maintenance, occurs with very low frequency and may involve epigenetic modifications of the incoming viral genomes. Indeed, rare cases in which cells of endothelial origin (TIVE and SLK) support stable latency after de novo infection have been reported (3, 9, 22).
To quantitatively access these rare events, we examined the kinetics of TR-containing replicons within proliferating cell populations in the absence and presence of LANA under nonselective conditions. Contrary to previous reports, we observed a significant effect of LANA on the short-term retention of KSHV replicons. In our model system, LANA improves plasmid retention twofold when provided in cis and fourfold when provided in trans. Additionally, long-term maintenance of KSHV replicons can be observed at low frequencies. Using this system in conjunction with colony formation assays, we show that the cis-regulatory elements conferring episome partitioning consist of multiple LANA binding sites within multiple TRs which function in a manner analogous to that of the FR element of EBV. Our data indicate that the early replication and partitioning events mediated by LANA are fundamental to initiating episome establishment and maintenance within dividing cells.
MATERIALS AND METHODS
Plasmids.
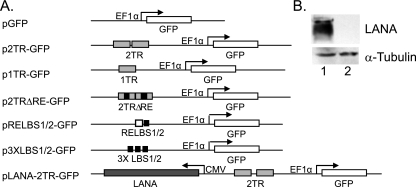
pGFP was constructed by inserting a GFP cassette from pHPT-GFP (kindly provided by Stanton Gerson, Case Western Reserve University) into pCRII (Invitrogen). GFP expression is driven by the EF1α promoter, which augments strong expression over long periods of time without being translationally silenced (56). p2TR-GFP contains two copies of TR in tandem (described in reference 28), while p1TR-GFP contains one 801-bp TR at NotI. p2TRΔ512-556 was derived from pTRΔ512-556 (described in reference 29). Expression of LANA, a 1,003-amino-acid full-length variant (20), is driven by a cytomegalovirus promoter. Plasmids containing RELBS1/2 or two or three sets of LBS1/2 were constructed from oligonucleotides (Integrated DNA Technologies, Inc.). Puromycin-containing plasmids are based on pPur (Invitrogen). pPur-DS-FR and pPur-DS were gifts from Ashok Aiyar. TRs or derivatives were inserted at the PvuII site of pPur or pPur-DS. All constructs were confirmed by restriction enzyme digestion and/or sequencing and are illustrated in Fig. 1 and 6.
FIG. 1.
KSHV replicons. (A) Schematic of plasmid constructs used in retention assays. GFP is inserted into pCRII (Invitrogen) at XhoI to XbaI; TRs or derivatives are at NotI; LANA is at EcoRV. Plasmid p2TRΔRE-GFP was derived from pTRΔ512-556 (described in reference 29) and contains two copies of TR in tandem, each lacking the G+C-rich region encompassing RE directly adjacent to LBS1/2. pRELBS1/2-GFP contains 71 bp of the minimal replicator inserted HindIII to NotI, while p3XLBS1/2-GFP contains three sets of minimal LBS1/2 at NotI in tandem. (B) LANA expression at 24 h in 293 cells transfected with 1 μg pLANA-2TR-GFP (lane 1) or p2TR-GFP control (lane 2). α-Tubulin was used as a loading control.
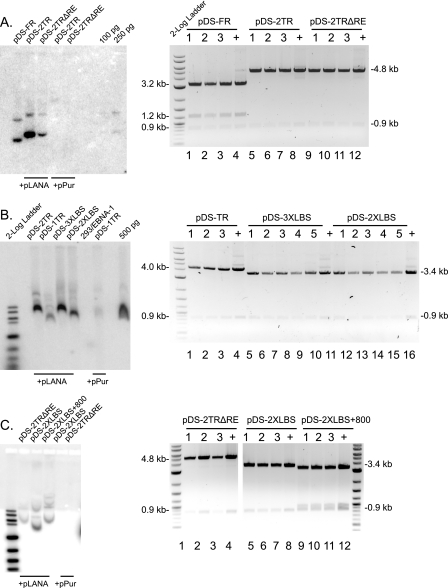
FIG. 6.
Hybrid origins are maintained as episomes. (A to C, left panels) Southern blot analysis of DNA extracted by the Hirt method from 293/EBNA1 cell pools transfected with hybrid origins 15 to 17 days following puromycin selection. Prior to Hirt extraction, cells were washed twice in PBS. Undigested episomal DNA was detected using pPurTR-TRΔRE as a probe. (A to C, right panels) Plasmid rescue assay. Ten percent of each Hirt extract was digested for >48 h with DpnI and retransformed into E. coli. Hybrid origins cotransfected with pLANA yielded a significant number of bacterial colonies (∼300 to 600), while plasmids cotransfected with pPur yielded less than 5 or none. Three to five individual colonies for each hybrid origin cotransfected with pLANA were selected for restriction enzyme analysis. DNA was digested with NcoI, and expected fragment sizes are indicated; +, 250 ng control plasmid DNA.
Cell lines and transfections.
BJAB, an EBV/KSHV-negative Burkitt's lymphoma B-cell line, and BJAB/TetOn/ORF73, previously described (2), were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and 5% penicillin-streptomycin. For plasmid retention assays, cells were kept in the log phase of growth (105 to 8 × 105 cells/ml) at all times. Cell counts were determined by trypan blue exclusion. Seventy-two hours prior to transfection, BJAB/TetOn/ORF73 was induced to express LANA by the addition of 1 μg/ml doxycycline (Dox). BJAB cells were transfected either by traditional electroporation methods in Opti-MEM reduced serum medium (Invitrogen) using 15 μg of plasmid DNA with 950 μF and 250 V (Bio-Rad Genepulser) or by nucleofection using 0.04 fmol of plasmid DNA per 5 × 106 cells, solution T, program O-17, as per the manufacturer's instructions (Amaxa, Inc.). For colony formation assays, 293, 293/LANA, and 293/EBNA-1 cells were grown in supplemented Dulbecco's modified Eagle medium and transfected using Effectene (QIAGEN, Inc.) as per the manufacturer's protocol. Forty-eight hours posttransfection, cells were plated and 1 μg/ml puromycin (Calbiochem) was added.
Immunoblotting.
Whole-cell lysates were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore). Polyclonal rabbit anti-LANA (2), antitubulin (Oncogene Research), or mouse antiactin (Santa Cruz Biotechnology, Inc.) antibodies were used to detect proteins. Blots were developed with peroxidase-conjugated antibodies and an enhanced-chemiluminescence substrate (Pierce).
FACS and flow cytometry analysis.
To obtain clonal populations, cells were sorted into 96-well plates at 3 cells per well 48 h posttransfection (Elite ESP, Beckman Coulter). Photomicrographs of GFP-positive cells were taken with an inverted fluorescence microscope (Nikon). For short-term maintenance assays, cells were batch sorted at 18 to 22 h posttransfection, achieving >95 to 99% GFP-positive cell populations (FACS-Diva; Becton Dickinson), and resuspended in complete medium at equal cell densities. To measure GFP expression, cell aliquots were fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) at selected time points and stored at 4°C until the time of analysis. GFP percentages are based on gated viable cells, using CellQuest software, and are always compared to a BJAB negative control (FACSCalibur; Becton Dickinson). Episome loss follows first-order exponential decay, defined by the equation N(t) = N0e−kt, where t is the number of cell generations and k is the rate of plasmid loss, defined as the percentage of cells losing plasmids per cell generation as measured by GFP expression. Since cells were sorted by fluorescence-activated cell sorting (FACS) at the beginning of each experiment, N0 = 100%. This equation model is used to calculate k values at 5 days posttransfection, shown in Table 1.
TABLE 1.
Average loss rates of KSHV replicons
| Plasmid | k (% rate loss/generation) ± SDa | No. of expts |
|---|---|---|
| pGFP | 27.8 ± 0.3 | 4 |
| p1TR-GFP | 26.6 ± 0.2 | 2 |
| p2TR-GFP | 25.6 ± 0.3 | 6 |
| pLANA-2TR-GFP | 13.6 ± 0.2 | 6 |
| p1TR-GFP+Dox | 8.2 ± 0.2 | 4 |
| p2TR-GFP+Dox | 5.9 ± 0.2 | 4 |
Average loss rates per cell generation (k) with standard deviations are calculated from days zero to four post-FACS.
PCR and Southern hybridization.
Episomal plasmid DNA was prepared from cells using either the method of Hirt (27) as described previously (20) or a modified Hirt extraction method as described previously (4). Hirt extracts were resuspended in 50 μl of distilled H2O containing RNase A or 50 μl of 10 mM Tris-EDTA, pH 8.0, for the modified protocol. PCR amplification was performed using primers specific for the GFP gene (fwd, 5′-AGATCCGCCACAACATCGAG-3′; rev, 5′-CCATGCCGAGAGTGATCC-3′) and products visualized on 1.5% agarose gels.
For Southern blot analysis, extracted DNA was loaded directly into wells of 0.8% agarose gels and transferred to Immobilon-Ny+ membranes (Millipore) following electrophoresis. A radioactive probe was prepared by random-prime labeling plasmid DNA (Amersham Biosciences) with [32P]dCTP and purifying with quick-spin columns (Roche). Hybridization and washing were performed as described previously (28). Following hybridization, Southern blots were exposed to a phosphor screen and signals captured on a PhosphorImager using ImageQuant software (Molecular Dynamics).
DpnI PCR-based replication assays.
Ten percent of DNA extracted by the Hirt method was digested with 70 U DpnI (New England Biolabs) at 37°C for >72 h to eliminate bacterially methylated input DNA. Plasmid DNA which has undergone at least two rounds of DNA synthesis in eukaryotic cells is resistant to DpnI cleavage. As a control for input, equivalent amounts of extract were subjected to the same buffer conditions in the absence of enzyme. To detect DpnI-resistant species, digests were heat inactivated and an aliquot of each sample was subjected to 27 to 30 PCR amplification cycles using GFP-specific primers.
Colony formation assays.
Cells were cotransfected with 0.7 μg replicon DNA and 0.3 μg pLANA or pPur. Twenty-four hours posttransfection, cells were washed twice in PBS, trypsinized, and plated at equal densities (2 × 104, 5 × 104, 1 × 105, or 2 × 105 cells) in 10-cm plates. Cells were grown in the presence of puromycin for more than 2 weeks. To visualize colonies, cells were fixed in 80% methanol and stained in 30% methanol in PBS containing 0.1% crystal violet.
Plasmid rescue assay.
DpnI-digested, Hirt-extracted DNA at >15 days posttransfection was transformed into chemically competent DH5α and plated onto ampicillin LB plates. Bacterial colonies were analyzed by NcoI restriction enzyme digestion following plasmid purification.
RESULTS
LANA significantly increases retention of KSHV replicons in absence of antibiotic selection.
Previous reports indicate that TR plasmids are maintained in the presence of LANA only under selective conditions (5, 22). The fact that latent viral genomes are readily detectable within 36 h postinfection (9) suggests that LANA has a significant effect on episome establishment early after infection. To examine these early events in a quantitative manner, we measured the short-term retention rates of GFP-expressing replicons in the absence of selective agents.
First we constructed a series of KSHV replicons containing a GFP reporter gene, allowing us to track cells that maintain replicons by microscopy and flow cytometry (Fig. 1A). The complete replicon, pLANA-2TR-GFP (11.3 kb), contains all required viral elements for episome replication and maintenance. Derivatives used for controls include p2TR-GFP (7.0 kb), lacking the LANA expression cassette, and pGFP (5.4 kb), lacking both cis (TR) and trans (LANA) elements. LANA expression from pLANA-2TR-GFP was confirmed by Western blot analysis (Fig. 1B).
To investigate replicon kinetics at early time points after transfection, we implemented a novel transfection method which enables us to transfect cells at high efficiencies while preserving cell viability (Amaxa, Inc.). To further recapitulate events after de novo infection, we introduced the smallest amount of DNA possible, an optimized 5,000 copies per cell (∼150 to 200 ng per 5 million cells). Equimolar amounts of plasmid DNA were introduced into BJAB cells, which represent a B-cell lymphoma line previously shown to replicate TR plasmids in the presence of LANA (23). Eighteen hours posttransfection, cells were FACS sorted and equal numbers of GFP-positive cells seeded and maintained in nonselective medium. Cells were analyzed every 24 h for GFP expression, and the percentages of GFP-positive cells were plotted over time.
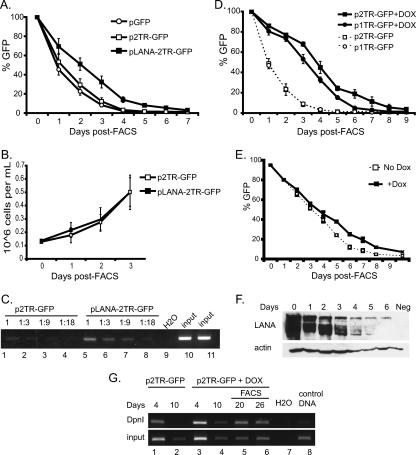
GFP expression in pGFP- and p2TR-GFP-transfected populations followed similar kinetics, decreasing rapidly to about 1% within 5 days of FACS (Fig. 2A). While GFP expression in cells transfected with pLANA-2TR-GFP also declined, the presence of LANA significantly slowed plasmid loss. At 4 days post-FACS, 13.9% of cells were GFP positive, compared to less than 3% for the controls. By 7 days post-FACS, the level of GFP expression stabilized at 3% ± 0.5% for pLANA-2TR-GFP (compared to 1.2% ± 0.3% for pGFP and 1.2% ± 0.1% for p2TR-GFP) and remained at this level indefinitely (Fig. 2A; also data not shown). To compare the retention ability for each plasmid, we calculated the rate of plasmid loss per cell generation (Table 1). With LANA expression, TR plasmids were lost at a rate of 13.6% ± 0.2% per cell generation. In contrast, control plasmids were lost at rates of 27.8% ± 0.3% (pGFP) and 25.6% ± 0.3% (p2TR-GFP) per generation. These data demonstrate that TR-containing replicons are retained two times more efficiently when LANA is provided in cis (Fig. 2A; Table 1).
FIG. 2.
LANA significantly increases the retention of TR plasmids. (A) Short-term retention of KSHV replicons. BJAB cells were transfected with 5,000 copies of plasmid DNA per cell using nucleofection (Amaxa, Inc.). Twenty hours posttransfection, cells were subjected to FACS and GFP expression monitored by flow cytometry. Each curve represents the average for four independent transfections. As determined by Student's t test, P values were <0.01 for pLANA-2TR-GFP compared to either p2TR-GFP or pGFP. (B) Growth of FAC-sorted cells was monitored by trypan blue exclusion. (C) LANA expression in cis facilitates replication of KSHV replicons. Hirt extracts at 4 days were prepared from equal numbers of BJAB cells transfected with p2TR-GFP (lanes 1 to 4 and 10) or pLANA-2TR-GFP (lanes 5 to 8 and 11). DpnI-resistant DNA or undigested DNA (input lanes) was PCR amplified using GFP-specific primers to amplify a product of 203 bp. Digests were diluted 1, 1:3, 1:9, or 1:18 prior to PCR. (D) LANA in trans enhances replicon retention. BJAB or Dox-induced BJAB/TetOn/ORF73 cells (+Dox) were transfected with 5,000 copies of plasmid DNA per cell, and GFP expression was monitored post-FACS. Shown is the average of two independent transfections. As determined by Student's t test, P values were <0.01 for BJAB results versus results with BJAB/TetOn/ORF73. (E) LANA expression is required for retention of GFP-expressing replicons. GFP-positive BJAB/TetOn/ORF73 cells transfected with 5,000 copies of p2TR-GFP per cell were grown in the presence (+Dox) or absence (No Dox) of Dox following FACS. Transfections were performed in duplicate. As determined by Student's t test, P values were <0.01 for +Dox results compared to No Dox results between days five and eight. (F) Western blot analysis of LANA expression in BJAB/TetOn/ORF73 cells in the 6 days following release from Dox induction. BJAB lysate is shown as a negative control; actin is shown as a loading control. (G) Long-term replication of KSHV replicons. BJAB or induced BJAB/TetOn/ORF73 (Dox) cells were transfected with 50,000 copies of p2TR-GFP per cell. At day 10 posttransfection, GFP-positive LANA-expressing cells were subjected to FACS. DNA extracted by the Hirt method was prepared 4, 10, 20, and 26 days posttransfection. Equal amounts of DpnI-resistant DNA and input DNA corresponding to 2,000 cell equivalents were PCR amplified using GFP-specific primers.
Previously it was shown that LANA targets the wnt/β-catenin pathway (19), thus promoting S phase. To rule out that LANA-expressing BJAB cells have a growth advantage, thereby affecting our measurements of replicon maintenance, we monitored growth of FAC-sorted BJAB cells but observed no differences (Fig. 2B).
To confirm that the observed differences in GFP expression are due to LANA activity, we tested plasmid replication. Episomal Hirt-extracted DNA prepared 4 days posttransfection was subjected to DpnI digestion for more than 72 h and subsequently amplified by PCR. DpnI-resistant species were readily detectable in cells transfected with pLANA-2TR-GFP (Fig. 2C, lanes 5 to 8) but not with p2TR-GFP (lanes 1 to 4), demonstrating LANA-dependent replication.
To determine whether provision of LANA in trans would enhance replicon retention in our model system, we monitored plasmid kinetics in LANA-inducible BJAB/TetOn/ORF73 cells (described in reference 2). Cells were transfected with plasmids containing either one or two copies of TR. Following FACS, we again observed a rapid loss of GFP expression from LANA-negative cells (Fig. 2D). Provision of LANA in trans significantly reduced the loss of TR plasmids, and plasmids containing only a single copy of TR behaved similarly to p2TR-GFP (Fig. 2D,). The loss rate of p2TR-GFP was 5.9% in LANA-expressing cells, compared to 26.9% in BJAB controls (Table 1), resulting in a fourfold increase in retention. This retention is two times higher than when LANA was provided in cis (Fig. 2A). These data show that provision of LANA in cis or in trans significantly enhances the retention of TR plasmids in proliferating cells under nonselective conditions.
To rule out the potential effect of plasmid size on retention and stability, we introduced p2TR-GFP into BJAB/TetOn/ORF73 cells grown in Dox-plus medium for 72 h. Following FACS, cells were reseeded in either Dox-plus or Dox-minus medium. Cells released from Dox induction retained GFP expression less efficiently than cells maintained in Dox-plus medium (Fig. 2E). This decrease in plasmid retention was tightly linked to the loss of LANA expression as monitored by Western blotting (Fig. 2F). We have also tested a replicon containing an EBV replication origin (pDS-GFP) and observed no differences in plasmid kinetics in the presence or absence of LANA (data not shown), demonstrating that LANA has no effect on plasmids that lack TR. Thus, GFP expression correlates directly with retention of TR-containing plasmids. Together these data suggest that LANA increases viral episome retention early after infection.
KSHV replicons can be episomally maintained long term in rare subpopulations in the absence of drug selection.
Several reports have shown that KSHV and EBV replicons are unstable and cannot be maintained long term in the absence of antibiotic selection (22, 39); however, we have observed GFP-expressing colonies in 293/LANA cells transfected with p2TR-GFP after 15 days posttransfection and replicating TR plasmids in GFP-positive LANA-expressing BJAB cells as long as 4 weeks posttransfection (data not shown). Additionally, we have observed that the percentage of GFP-positive cells stabilizes at ∼3% within 7 days post-FACS for either cells transfected with pLANA-2TR-GFP or Dox-induced cells transfected with p2TR-GFP (Fig. 2). Thus, we hypothesized that rare cells within a population are competent in establishing episome maintenance and such events occur at a low frequency.
To demonstrate long-term LANA-dependent replication of TR-containing plasmids, BJAB or Dox-induced BJAB/TetOn/ORF73 cells were transfected with p2TR-GFP. On various days, plasmid DNA was extracted by the Hirt method and digested with DpnI and resistant species amplified by PCR. At 10 days posttransfection, BJAB cells were ∼0.2% GFP positive and tested negative for DpnI-resistant DNA while LANA-expressing cells were ∼13% GFP positive and exhibited DpnI-resistant replicated plasmid forms (Fig. 2G, lanes 2 and 4). To enrich for cells maintaining replicons, GFP-expressing LANA-positive cells were subjected to FACS at day 10 and Hirt extracts prepared 10 and 16 days later for PCR analysis. At 20 and 26 days posttransfection (10 and 16 days post-FACS), DpnI-resistant plasmids continued to be present in LANA-expressing cells. Interestingly, at these later time points, the intensity of DpnI-digested bands is similar to that of input DNA, suggesting that LANA-positive cells are replicating and maintaining p2TR-GFP as stable episomes (Fig. 2G, lanes 5 and 6).
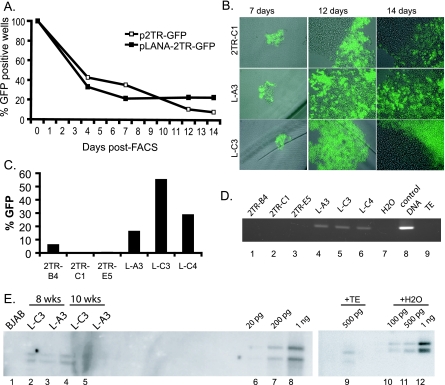
We next examined plasmid maintenance on an individual cell basis. BJAB cells were transfected with p2TR-GFP or pLANA-2TR-GFP, and single cells were FACS sorted into 96-well plates. At day 4 post-FACS, 21 of 49 wells contained GFP-positive, viable cells for p2TR-GFP and 19 of 52 wells for pLANA-2TR-GFP were GFP positive. After 10 days, three wells for p2TR-GFP and seven wells for pLANA-2TR-GFP remained GFP positive (Fig. 3A).
FIG. 3.
Subpopulations maintain replicons as episomes long term in the absence of selection. (A) BJAB cells were subjected to FACS in 96-well plates 48 h posttransfection at 3 cells per well and scored for GFP expression by fluorescence microscopy. The percentage of GFP-positive wells was determined from the ratio of GFP-positive wells to wells containing viable cells. (B) Photomicrographs of 3 clones from 96-well plates at 7, 12, and 14 days post-FACS. (C) Analysis of GFP expression by flow cytometry for three p2TR-GFP (2TR) and three pLANA-2TR-GFP (L) subpopulations at >2.5 months. (D) PCR analysis of DNA extracted by the Hirt method at 2.5 months post-FACS for subpopulations transfected with p2TR-GFP (lanes 1 to 3) or pLANA-2TR-GFP (lanes 4 to 6). Fifty picograms of p2TR-GFP plasmid DNA was used as a positive control (lane 8); TE, Tris-EDTA buffer (lane 9). (E) Southern blot analysis of two pLANA-2TR-GFP populations at 8 weeks (lanes 2 and 3) and 10 weeks (lanes 4 and 5) posttransfection. Episomal DNA prepared using a modified Hirt extraction protocol from 2 × 106 long-term-cultured cells was detected using p2TR-GFP as a probe. Episomal DNA is observed in two forms: a top band, corresponding to open circular, and a lower band, corresponding to covalently closed circular. Indicated amounts of pLANA-2TR-GFP control DNA are at right as standards for quantification (lanes 6 to 8). At far right, control DNA was loaded in TE Hirt resuspension buffer or water to show differences in migration due to buffer conditions (lanes 9 to 12).
At 14 days, three subpopulations from pLANA-2TR-GFP and all three p2TR-GFP subpopulations were triturated and expanded and remained under continuous microscopic observation for more than 2 months (Fig. 3B). LANA-expressing populations sustained a much higher percentage of GFP-expressing cells, ranging from 16.3% to 55.3% GFP positive, while expression in control populations was significantly lower, ranging from 0.3% to 6.2% (Fig. 3C), suggesting that cells were maintaining KSHV replicons.
To verify that pLANA-2TR-GFP plasmids were maintained as episomes, DNA extracted by the Hirt method was analyzed 10 weeks posttransfection by PCR. Cells transfected with p2TR-GFP (Fig. 3D, lanes 1 to 3) were negative, indicating that the small number of GFP-expressing cells in these populations harbor integrated plasmids. However, cells transfected with pLANA-2TR-GFP (lanes 4 to 6) were positive, indicating the presence of episomal DNA. To confirm these results in a PCR-independent fashion, we performed Southern blot analysis. DNA extracted by the Hirt method at 8 or 10 weeks post-FACS tested positive (Fig. 3E), demonstrating that these subpopulations (L-A3 and L-C3) stably maintained replicons. Semiquantitative Southern blot analysis revealed low plasmid copy numbers, averaging 3 to 10 molecules per cell.
These results demonstrate that in the presence of LANA, TR-containing plasmids can be maintained as episomes at a low frequency over a long period of time in dividing cells. Additionally, these data suggest that a small fraction of cells is competent to support the establishment of episomes in a LANA-dependent fashion under nonselective conditions.
Replication-deficient TR plasmids are initially retained in the presence of LANA but with less efficiency.
Episome maintenance can be described as the sum of two processes: replication and partitioning. EBV oriP contains two distinct sequence elements for these processes. DS conveys EBNA-1-dependent replication, while FR facilitates partitioning (43, 51, 52, 71). For KSHV, all required sequences are located within the TRs (5). The minimal replicator has been mapped to a 71-bp region (nucleotides 539 to 610) containing LBS1/2 and an adjacent 32-bp G+C-rich RE and confers replicative activity at about 25% that of wild-type (wt) TR (29, 67). Sequence requirements for LANA-dependent partitioning, on the other hand, have not been investigated. Several reports suggest that multiple TRs are needed for efficient long-term maintenance (6, 22); however, whether LBS1/2 alone or additional TR sequences contribute to partitioning is not known.
To address this question, we first asked whether plasmids containing LBS1/2 but not RE, and therefore unable to replicate, could be retained in LANA-expressing cells. We compared retention of wt TR (p1TR-GFP), the minimal replicator (pRE-LBS1/2-GFP), or a plasmid containing three sets of LBS1/2 in tandem (p3XLBS1/2-GFP) in BJAB and LANA-inducible BJAB/TetOn/ORF73 cells.
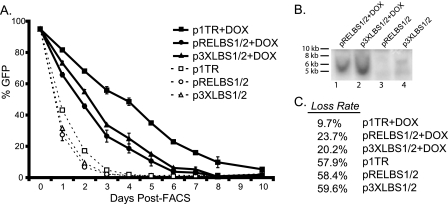
In LANA-negative cells, GFP expression was lost rapidly irrespective of the transfected plasmids (Fig. 4A). In congruence with data shown above, LANA expression greatly reduced the loss of wt TR (Fig. 4A). While pRE-LBS1/2 and p3XLBS1/2, which does not replicate, were lost faster than wt TR, the presence of LANA significantly reduced the loss of these plasmids (Fig. 4A). Loss rates calculated for each plasmid showed that LBS1/2-containing plasmids were retained approximately 2.5 to 3 times more efficiently with LANA (Fig. 4C). By performing Southern blot analysis of DNA extracted by the Hirt method 96 h posttransfection, we detected pRE-LBS1/2 and p3XLBS1/2 only in LANA-expressing cells (Fig. 4B, lanes 1 and 2). Importantly, by 72 h posttransfection, more than 95% of the transfected DNA is degraded in the absence of LANA (data not shown). Thus, early on, LANA seems to stabilize LBS1/2-containing plasmids in the absence of DNA replication.
FIG. 4.
Replication-deficient plasmids exhibit moderate retention in the presence of LANA. (A) BJAB (dashed lines) or LANA-positive BJAB/TetOn/ORF73 (+Dox) cells (solid lines) were transfected with equimolar amounts of plasmid DNA corresponding to 5,000 copies per cell, subjected to FACS at 20 h posttransfection, and monitored by flow cytometry. Transfections were done in duplicate. (B) Replication-deficient plasmids are retained as episomes in LANA-expressing cells. DNA extracted by the Hirt method at 4 days posttransfection was analyzed by Southern blotting. Undigested episomal DNA was detected using pGFP as a probe. (C) Loss rates for each plasmid were calculated between 1 and 4 days post-FACS. According to Student's t test, P values were <0.01 for LANA-positive versus BJAB cells and <0.01 for p1TR compared to pRELBS1/2 and p3XLBS1/2.
Multiple LBS1/2 function as a cis-partitioning element analogous to FR of EBV oriP.
Based on our data shown in Fig. 4, we hypothesized that although replication and partitioning are LANA dependent, the two steps occur independently. To directly test this, we uncoupled replication and partitioning elements by constructing hybrid origins which contain the DS element of EBV oriP and various TR mutants in the background of pPur (Fig. 5A) and performed colony formation assays. Hybrid origins should replicate in an EBNA-1-dependent fashion but require LANA for efficient partitioning. Similar hybrid origins have successfully been utilized to separate cis elements of other DNA tumor viruses (63).
FIG. 5.
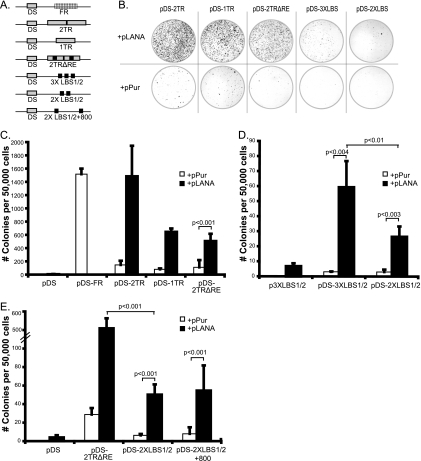
LBS1/2 functions as a cis-partitioning element. (A) Schematic representation of hybrid origin constructs used in colony formation assays. Plasmids contain pPur as the vector backbone and EBV DS as a replication origin. EBV FR is replaced with various TRs or TR mutants, all oriented in tandem. (B) Hybrid origins were cotransfected with a puromycin plasmid expressing full-length LANA (pLANA) or empty vector (pPur) into 293/EBNA1 cells. At 24 h posttransfection, cells were washed in PBS, trypsinized, and plated at equal densities. Puromycin selection was applied 48 h posttransfection. Crystal violet staining was performed 2 weeks following selection, and colonies are shown for indicated plasmids. (C to E) Enumeration of puromycin-resistant colonies. Graphs represent the average for at least four plates from two independent transfections. Solid bars indicate cotransfection with pLANA; open bars indicate cotransfection with pPur. Statistical P values are reported according to Student's t test.
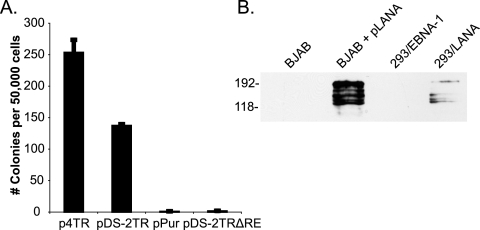
Starting with pDS-FR (wt EBV oriP), we replaced the FR element with either one or two TRs (pDS-1TR and pDS-2TR) or two TRs both lacking RE (p2TRΔRE). 293 cells stably expressing EBNA-1 (a kind gift from Ashok Aiyar) were cotransfected with each hybrid origin and either pPur-LANA or pPur as a control. After transfection, equal numbers of cells were seeded and selected with puromycin, and after 2 weeks, outgrowing colonies were stained and enumerated. The number of outgrowing colonies is a direct measure of the efficiency of long-term maintenance. As a negative control, we tested a replication-defective plasmid, p2TRΔRE, in 293/LANA cells and observed no colonies (see Fig. 7A). In congruence with previous reports, pDS-FR produced 1,516 ± 83 colonies per 50,000 plated cells while pDS did not form colonies (Fig. 5C) (26). In the absence of LANA, pDS-2TR and pDS-1TR gave a low number of colonies but produced 1,483 ± 461 and 645 ± 51 colonies, respectively, when cotransfected with pLANA (Fig. 5C), demonstrating that these assays allow us to monitor LANA-dependent maintenance. Importantly, cotransfection of pDS-2TRΔRE with pLANA produced 506 ± 109 colonies, proving that LANA can partition episomal DNA that is replicated in an EBNA1-dependent fashion.
FIG. 7.
Long-term maintenance is more efficient with multiple TRs. (A) 293/LANA cells were transfected with the plasmids indicated, and colony formation assays were performed as described in Methods and in the legend to Fig. 5. Puromycin-resistant colonies were quantified following crystal violet staining. The graph represents the average for four plates from two transfections. (B) LANA expression by Western blot analysis in BJAB cells transfected with pLANA and in 293/LANA cells. BJAB and 293/EBNA1 are shown as the negative controls. Lysates are from 1 × 105 cells.
To address whether LANA binding sites alone can confer partitioning, we tested hybrid origins containing either two or three copies of LBS1/2 (pDS-2XLBS1/2 and pDS-3XLBS1/2) (Fig. 5A). Both plasmids formed colonies in a LANA-dependent fashion, and increasing the number of LBS1/2 significantly increased the number of colonies produced (Fig. 5B and D). These observations indicate that LBS1/2 within the TR functions as a cis-partitioning element comparable to the EBV FR element.
We observed that the number of colonies formed from plasmids containing minimal LBS1/2 was greatly reduced from that with full-length TR. For instance, while pDS-2TRΔRE generated more than 500 colonies, pDS-2XLBS1/2 produced 26 ± 6 colonies, which were strictly dependent on the presence of LANA (Fig. 5C and D). One possible explanation for this difference is the spacing between the sets of LANA binding sites within the viral genome. Spacing between adjacent EBNA-1 binding sites within EBV FR has been shown to be critically important for stable oriP plasmid maintenance (26). In both wt p2TR and pDS-2TRΔRE, consecutive sets of LBS1/2 are spaced about 800 bp apart. In contrast, for pDS-2XLBS1/2, the sets of LANA binding sites are directly adjacent to each other, spaced by only 6 bp. Accordingly, we generated pDS-2XLBS1/2+800 by inserting an 808-bp spacer of unrelated DNA between the two sets of LBS1/2 (Fig. 5A); however, no significant differences in the number of colonies were observed (Fig. 5E). In summary, these results suggest that while multiple copies of LBS1/2 convey LANA-dependent partitioning, sequences outside of the minimal replicator region may contribute to the efficiency of this process.
Hybrid origins containing LBS1/2 are episomally maintained.
To confirm that all hybrid origins which formed colonies were indeed maintained as episomes, we prepared Hirt extracts from puromycin-resistant cell pools and analyzed episomal DNA by Southern blotting. Consistently, we detected strong signals for hybrid origins cotransfected with pLANA, while those transfected with pPur showed no signal (Fig. 6A, B, and C, left panels).
To verify hybrid origins had not undergone genetic rearrangements, Hirt extracts were subjected to DpnI digestion and retransformed into Escherichia coli. Restriction enzyme analysis showed that rescued plasmids were the correct size (Fig. 6A, B, and C, right panels). Thus, hybrid origins containing minimal LBS1/2 are maintained as episomes in dividing cells. These data formally prove that LBS1/2 sites within the TR function in a manner analogous to that of the FR element of EBV.
The number of LANA binding sites within TR affects the outcome of stable plasmid maintenance.
KSHV viral genomes contain between 35 and 45 copies of TR (36). Our results shown in Fig. 5 and those of previous reports indicate that plasmids bearing multiple TRs are more efficient in episome maintenance (6, 22). To test this in a quantitative manner, we examined long-term maintenance of plasmids containing either two or four copies of TR in 293 cells that stably express LANA (Fig. 7B). We observed a twofold increase in the number of outgrowing colonies with plasmids containing four TRs compared to that with plasmids containing only two TRs (Fig. 7A). Similarly, an increase in TR copies from one to two yielded more colonies (Fig. 5B and C). These data show that multiple TRs enhance the efficiency of long-term episome maintenance.
DISCUSSION
In this study, we used a GFP reporter replicon system to follow the establishment and maintenance of TR-containing plasmids in a LANA-dependent fashion. Additionally, we generated EBV/KSHV hybrid origins to define cis-regulatory elements required for LANA-mediated episome partitioning.
LANA significantly increases the retention rate of TR-containing plasmids early after transfection.
To monitor kinetics of KSHV replicons in the absence of selection, we used nucleofection in combination with FACS. Both efficient plasmid delivery and rapid GFP expression allowed us to examine retention events during the first few cell divisions in a highly repeatable and quantifiable fashion. TR-containing replicons were retained two to four times more efficiently in the presence of LANA (Fig. 2), and such retention was conferred by both active DNA replication and LANA-mediated partitioning (Fig. 2 and 4). Surprisingly, we found that TR mutants incapable of replication exhibited measurable retention rates in the presence of LANA, suggesting that binding of LANA to TR also stabilizes and/or protects incoming DNA from degradation (Fig. 4B). Importantly, GFP expression correlated well with the presence of episomal DNA, which was monitored by both PCR and Southern blot analysis (Fig. 2 and 3). The fact that we observed a loss of GFP expression from LANA-expressing cells can be explained in at least two ways. Either cells maintaining replicons are outgrown by those that do not or the stabilization and establishment of replicons occurs over several cell divisions. The latter is more likely, since resorting of LANA-expressing cells at 2 or 4 days post-FACS results in similar loss kinetics up until approximately day seven, when the percentage of GFP-expressing cells becomes constant (Fig. 2; also data not shown). Thus, these initial experiments reveal that replication and partitioning of KSHV episomes, although LANA dependent, occur independently and that events early after de novo infection, here recapitulated by transfection, are critical for episome establishment.
Episome maintenance of TR plasmids can occur in the absence of antibiotic selection.
Analysis of clonal populations revealed that a small percentage of cells retain KSHV replicons for several months posttransfection in the absence of antibiotic selection. These cells maintain episomal DNA in the presence of LANA as, shown by both Southern blotting and PCR analysis (Fig. 3). Although the frequency of clones was low (<7%), these experiments suggested that LANA and TR can indeed confer episome maintenance in dividing cells under nonselective conditions. Furthermore, this low establishment frequency mimics that observed following de novo infection in vitro (3, 9).
Episome maintenance is a long-standing paradigm for gammaherpesvirus latency (40) but lately has been brought into question. Most cells infected in vitro with KSHV fail to establish stable latency and lose viral genomes as they proliferate (9, 35). Additionally, long-term maintenance of KSHV replicons was reported to require antibiotic selection (22). In response to these observations, Grundhoff et al. proposed a new model potentially explaining the fact that the majority of cells within KS tumors are infected but lack an efficient mechanism to segregate latent genomes. Rather than stable episome maintenance, a minority of cells could spontaneously reactivate to produce progeny virions, thereby continuously replenishing the pool of infected cells (22). Indeed, early in situ hybridization studies showed that a small number of cells within KS tumors express lytic markers (64).
This reasoning, however, leaves out an important genetic argument which stems from the fact that all gammaherpesviruses encode origin-binding proteins and have cis-regulatory elements that convey OBP-dependent origin activity. Additionally, stable episomal maintenance is observed in lymphoblastoid cell lines and Burkitt's lymphoma- and PEL-derived cell lines (11, 12, 24, 54). LANA knock-down experiments with PEL cells result in decreased KSHV copy numbers (21), demonstrating that the maintenance of episomal DNA, even in PELs, requires LANA. Likewise, KSHV bacmids, in which LANA expression is genetically disrupted, are no longer maintained in proliferating cells even under selective conditions (73).
Based on these observations and our data, we propose that the fate of the incoming viral DNA early after de novo infection is dependent upon robust expression of LANA. Interestingly, recent reports on viral gene expression profiling of de novo-infected endothelial cells show that there is a competition between ORF50 and LANA expression (33). Moreover, both proteins can regulate their counterpart promoters (31, 37, 38).
The presented data here, together with the outgrowth of a small number of stably infected KSHV-positive endothelium-derived SLK and human umbilical vein endothelial cells reported by the Ganem lab and us (3, 9, 22), suggest that a crucial event in the establishment of episome maintenance is the initial retention and stabilization of episomes by LANA. Elegant experiments by the Sudgen and Ganem labs demonstrated that stable episomes are genetically intact and cells in which episomes are established are also not genetically altered, since newly introduced episomes are lost as rapidly from stable cells as from naive cells. Hence, it was proposed that epigenetic modifications in cis occur for episome establishment (22, 39).
In this context, LANA interacts with a variety of chromatin components (8, 34, 41, 46, 55). Additionally, EBV latent genomes are packaged into nucleosomes (60), and it is known that KSHV TR is organized into nucleosomes, at which cell cycle-dependent histone modifications occur for initiation of DNA replication (65). Thus, the chromatin status of an episome is likely an important determinant in viral latency. Interestingly, recent work has shown that LANA can recruit Dnmt3a, a de novo methyltransferase, to cellular and viral promoters, resulting in hypermethylation and subsequently transcriptional silencing (59). We propose that the interaction of LANA with DNA methyltransferases also contributes to episome retention early after infection. In this model, LANA would interact with the viral genome and recruit DNA methyltransferases, facilitating epigenetic modifications as an initial step in sequestering and/or associating viral genomes with chromatin. In congruence with this model, we have tested in vitro-methylated TR plasmids in maintenance assays and found that they did not yield colonies (data not shown), suggesting that LANA-mediated recruitment of chromatin-associated factors, including DNA methyltransferases, is an important initial step in stabilizing episomes. In summary, we propose that gammaherpesvirus OBPs support episome establishment within a cell population by reducing the loss of viral genomes from individual dividing cells early after infection, permitting epigenetic stabilization events to occur.
Partitioning elements of KSHV episomes.
While sequence requirements for LANA-dependent DNA replication have been characterized (29), cis-regulatory elements conferring partitioning are less defined. Both bovine papillomavirus minichromosome maintenance element and EBV FR facilitate chromosome attachment and maintenance as part of hybrid origins containing a polyomavirus replication origin, showing that viral cis elements are interchangeable (63). Using EBV-DS/KSHV-TR hybrid origins, we demonstrate that (i) multiple copies of LBS1/2 form an array of OBP binding sites to confer LANA-mediated partitioning and (ii) replication and partitioning occur independently.
We observed that minimal LBS1/2 conferred partitioning but less efficiently than 2TRΔRE (Fig. 5). Similarly, we observed a reduction in the short-term retention of plasmids containing RELBS1/2 compared to results with wt TR (Fig. 3). These data indicate that sequences outside of LBS1/2 enhance episome maintenance. TR contains binding sites for several cellular proteins, including PARP1 (45), and transcription factors, such as Oct-1 and Sp1 (29, 68), as well as a variety of chromatin-associated proteins (62). Additionally, a low-affinity LANA binding site within TR has been demonstrated in vitro (17). Alternatively, the observed differences may be due to a disruption in chromatin architecture surrounding LBS1/2. Indeed, nucleosome positioning and chromatin remodeling are important factors in viral DNA replication (65, 74) and likely have a role in episome partitioning/tethering. In summary, our results suggest that while other TR-binding proteins may not be essential for partitioning, they may enhance episome stability.
Uncoupling replication and partitioning.
Our data indicate that replication and partitioning play compensatory roles in retention of viral episomes early after infection. Two lines of evidence support this observation. First, plasmids containing either RELBS1/2 (replicating) or three sets of LBS1/2 (nonreplicating) exhibit similar retention kinetics (Fig. 4). Thus, increasing the number of LBS1/2 from one to three somewhat compensates for the defect in replication, resulting in a retention rate comparable to that of a replication-competent plasmid. Second, DS plasmids with a single TR show the same maintenance efficiency as pDS-2TRΔRE, despite the presence of twice as many LANA binding sites within pDS-2TRΔRE (Fig. 5). These data suggest that transfected plasmids can be stabilized/retained either by replication or by LANA-dependent chromatin association prior to the cell entering S phase.
Interestingly, within EBV oriP, three copies of DS can replace FR in maintenance (70), suggesting that the mere presence of multiple OBP binding sites in an appropriate conformation conveys EBNA1-dependent tethering. While the number of EBNA-1 binding sites within FR does not affect DNA synthesis, at least four EBNA-1 sites spaced 14 bp apart are required for efficient oriP maintenance (26). For KSHV, plasmids bearing a single TR replicate with the same efficiency as those containing two TR, as shown by short-term replication assays (28). In contrast, for long-term maintenance, the number of TRs or more specifically the number of LBS1/2 directly affects the outcome of plasmid maintenance: two TRs are twice as efficient as one TR, and four TRs are twice as efficient as two TRs (Fig. 5 and 7). Since LANA oligomerizes when bound to LBS1/2 via its C-terminal DBD (20, 32, 57) and EBNA-1 dimerization is required for binding to its cognate sequences (10), the requirement of multiple OBP binding sites for efficient maintenance may be due in part to the higher-order structure which gammaherpesvirus OBPs form as they interact simultaneously with the viral episome via protein-DNA interactions and host chromatin via protein-protein interactions.
Chromosome association plays important roles in both the replication and tethering functions of EBNA-1 (30, 58) and LANA. A region within amino acid residues 5 to 22 of the LANA N terminus (CBS) is required for episome maintenance, interacts directly with core histones H2A and H2B, and can be substituted with histone H1 for maintenance of artificial replicons (8, 48, 61). The LANA C-terminal DBD supports DNA replication at about 20% of wt levels (28), and residues within the N-terminal CBS contribute to replication activity (7). In congruence, we have observed that replicons expressing only the LANA C terminus exhibit low retention rates, while addition of the N terminus partly rescues retention (data not shown), indicating that LANA-mediated tethering of KSHV replicons early after transfection brings DNA into a nuclear context where it is stabilized and can be efficiently replicated once the cell enters S phase. Importantly, LANA has been shown to overcome G1 cell cycle arrest (2) and cause nuclear accumulation of β-catenin (19), subsequently promoting S-phase entry. A fine mapping of TR sequences and examination of potential cellular proteins which may further support these early events is ongoing.
In summary, the dogma of gammaherpesvirus episome maintenance has shifted away from a simple picture in which episomes are segregated with absolute efficiency as is observed in lymphoblastoid cell lines or PELs. However, the data presented here clearly demonstrate that a mechanism exists which confers maintenance of KSHV episomes and that this multistep process is LANA dependent. Early on, various LANA activities may contribute to episome establishment by soliciting chromatin remodeling factors and/or facilitating epigenetic modifications of viral DNA. We like to suggest that the inefficiencies of episome maintenance observed in tissue culture are due in part to the experimental parameters utilized. For example, the dramatic reduction of transfected DNA in our model system allowed us for the first time to measure LANA-dependent retention rates of TR plasmids. Finally, we speculate that episome establishment may be more efficient in vivo than fast-growing, transformed cells in tissue culture due to the impact of LANA on cell signaling processes directly related to cell cycle control and S-phase induction, which can influence the establishment of latency. Therefore, combining our experimental system with the use of primary cells should increase our understanding of these important gammaherpesvirus-specific processes.
Acknowledgments
We thank Doug Smith at University of Florida Shands Cancer Center for help with FACS and Mike Sramkowski at Case Western Reserve University for help with FACS into 96-well plates. 293/EBNA-1 cells and oriP plasmids were kindly provided by Ashok Aiyar. Additionally, we thank Mark Samols and Soojin Han for critical reading of the manuscript.
R.L.S. is supported by National Cancer Institute Training Grant in Cancer Biology 5T32CA009126-30. This work was supported by CA88763 and CA097939 from the National Institutes of Health to R.R.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Aluigi, M. G., A. Albini, S. Carlone, L. Repetto, R. De Marchi, A. Icardi, M. Moro, D. Noonan, and R. Benelli. 1996. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi's sarcoma. Res. Virol. 147:267-275. [DOI] [PubMed] [Google Scholar]
- 2.An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862-3874. [DOI] [PubMed] [Google Scholar]
- 3.An, F. Q., H. M. Folarin, N. Compitello, J. Roth, S. L. Gerson, K. R. McCrae, F. D. Fakhari, D. P. Dittmer, and R. Renne. 2006. Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi's sarcoma-associated herpesvirus latency in vitro and in vivo. J. Virol. 80:4833-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arad, U. 1998. Modified Hirt procedure for rapid purification of extrachromosomal DNA from mammalian cells. BioTechniques 24:760-762. [DOI] [PubMed] [Google Scholar]
- 5.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 6.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbera, A. J., M. E. Ballestas, and K. M. Kaye. 2004. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 78:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, V. Joukov, J. C. Walter, K. Luger, and K. M. Kaye. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856-861. [DOI] [PubMed] [Google Scholar]
- 9.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochkarev, A., J. A. Barwell, R. A. Pfuetzner, E. Bochkareva, L. Frappier, and A. M. Edwards. 1996. Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin-binding protein, EBNA1, bound to DNA. Cell 84:791-800. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman, E. 2002. Epstein-Barr virus (EBV) and lymphomagenesis. Front. Biosci. 7:e58-e65. [DOI] [PubMed] [Google Scholar]
- 12.Cesarman, E. 2002. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 159:27-37. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 14.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 15.Chaudhuri, B., H. Xu, I. Todorov, A. Dutta, and J. L. Yates. 2001. Human DNA replication initiation factors, ORC and MCM, associate with oriP of Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 98:10085-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 17.Cotter, M. A., II, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 18.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 19.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 20.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey, A., J. Anderson, A. Papanastasiou, Y. Takeuchi, and C. Boshoff. 2005. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood 105:2510-2518. [DOI] [PubMed] [Google Scholar]
- 22.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerschmidt, W., and B. Sugden. 2004. Epstein-Barr virus sustains Burkitt's lymphomas and Hodgkin's disease. Trends Mol. Med. 10:331-336. [DOI] [PubMed] [Google Scholar]
- 25.Harrison, S., K. Fisenne, and J. Hearing. 1994. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. J. Virol. 68:1913-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebner, C., J. Lasanen, S. Battle, and A. Aiyar. 2003. The spacing between adjacent binding sites in the family of repeats affects the functions of Epstein-Barr nuclear antigen 1 in transcription activation and stable plasmid maintenance. Virology 311:263-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 28.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu, J., and R. Renne. 2005. Characterization of the minimal replicator of Kaposi's sarcoma-associated herpesvirus latent origin. J. Virol. 79:2637-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung, S. C., M. S. Kang, and E. Kieff. 2001. Maintenance of Epstein-Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains, which can be replaced by high-mobility group-I or histone H1. Proc. Natl. Acad. Sci. USA 98:1865-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong, J. H., J. Orvis, J. W. Kim, C. P. McMurtrey, R. Renne, and D. P. Dittmer. 2004. Regulation and autoregulation of the promoter for the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 279:16822-16831. [DOI] [PubMed] [Google Scholar]
- 32.Komatsu, T., M. E. Ballestas, A. J. Barbera, B. Kelley-Clarke, and K. M. Kaye. 2004. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology 319:225-236. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 37.Lan, K., D. A. Kuppers, S. C. Verma, and E. S. Robertson. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 78:6585-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan, K., D. A. Kuppers, S. C. Verma, N. Sharma, M. Murakami, and E. S. Robertson. 2005. Induction of Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency. J. Virol. 79:7453-7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leight, E. R., and B. Sugden. 2001. Establishment of an oriP replicon is dependent upon an infrequent, epigenetic event. Mol. Cell. Biol. 21:4149-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebowitz, D., and E. Kieff. 1993. Epstein-Barr virus, p. 107-172. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, New York, NY.
- 41.Lim, C., D. Lee, T. Seo, C. Choi, and J. Choe. 2003. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 278:7397-7405. [DOI] [PubMed] [Google Scholar]
- 42.Lim, C., H. Sohn, D. Lee, Y. Gwack, and J. Choe. 2002. Functional dissection of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 76:10320-10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lupton, S., and A. J. Levine. 1985. Mapping genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Mol. Cell. Biol. 5:2533-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 45.Ohsaki, E., K. Ueda, S. Sakakibara, E. Do, K. Yada, and K. Yamanishi. 2004. Poly(ADP-ribose) polymerase 1 binds to Kaposi's sarcoma-associated herpesvirus (KSHV) terminal repeat sequence and modulates KSHV replication in latency. J. Virol. 78:9936-9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottinger, M., T. Christalla, K. Nathan, M. M. Brinkmann, A. Viejo- Borbolla, and T. F. Schulz. 2006. Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80:10772-10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petre, C. E., S. H. Sin, and D. P. Dittmer. 2007. Functional p53 signaling in Kaposi's sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 81:1912-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raab-Traub, N. 1989. The human DNA tumor viruses: human papilloma virus and Epstein-Barr virus. Cancer Treat. Res. 47:285-302. [DOI] [PubMed] [Google Scholar]
- 50.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 51.Rawlins, D. R., G. Milman, S. D. Hayward, and G. S. Hayward. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42:859-868. [DOI] [PubMed] [Google Scholar]
- 52.Reisman, D., J. Yates, and B. Sugden. 1985. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol. Cell. Biol. 5:1822-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 55.Sakakibara, S., K. Ueda, K. Nishimura, E. Do, E. Ohsaki, T. Okuno, and K. Yamanishi. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78:7299-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salmon, P., V. Kindler, O. Ducrey, B. Chapuis, R. H. Zubler, and D. Trono. 2000. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood 96:3392-3398. [PubMed] [Google Scholar]
- 57.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sears, J., J. Kolman, G. M. Wahl, and A. Aiyar. 2003. Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by Epstein-Barr nuclear antigen 1. J. Virol. 77:11767-11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shamay, M., A. Krithivas, J. Zhang, and S. D. Hayward. 2006. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi's sarcoma-associated herpesvirus LANA. Proc. Natl. Acad. Sci. USA 103:14554-14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw, J. E., L. F. Levinger, and C. W. Carter, Jr. 1979. Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J. Virol. 29:657-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shinohara, H., M. Fukushi, M. Higuchi, M. Oie, O. Hoshi, T. Ushiki, J. Hayashi, and M. Fujii. 2002. Chromosome binding site of latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J. Virol. 76:12917-12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Si, H., S. C. Verma, and E. S. Robertson. 2006. Proteomic analysis of the Kaposi's sarcoma-associated herpesvirus terminal repeat element binding proteins. J. Virol. 80:9017-9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silla, T., I. Haal, J. Geimanen, K. Janikson, A. Abroi, E. Ustav, and M. Ustav. 2005. Episomal maintenance of plasmids with hybrid origins in mouse cells. J. Virol. 79:15277-15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stedman, W., Z. Deng, F. Lu, and P. M. Lieberman. 2004. ORC, MCM, and histone hyperacetylation at the Kaposi's sarcoma-associated herpesvirus latent replication origin. J. Virol. 78:12566-12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verma, S. C., T. Choudhuri, R. Kaul, and E. S. Robertson. 2006. Latency-associated nuclear antigen (LANA) of Kaposi's sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 80:2243-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma, S. C., T. Choudhuri, and E. S. Robertson. 2007. The minimal replicator element of the Kaposi's sarcoma-associated herpesvirus terminal repeat supports replication in a semiconservative and cell-cycle-dependent manner. J. Virol. 81:3402-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verma, S. C., K. Lan, T. Choudhuri, and E. S. Robertson. 2006. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen modulates K1 expression through its cis-acting elements within the terminal repeats. J. Virol. 80:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viejo-Borbolla, A., M. Ottinger, E. Bruning, A. Burger, R. Konig, E. Kati, J. A. Sheldon, and T. F. Schulz. 2005. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 79:13618-13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wysokenski, D. A., and J. L. Yates. 1989. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J. Virol. 63:2657-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yates, J., N. Warren, D. Reisman, and B. Sugden. 1984. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. USA 81:3806-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]
- 73.Ye, F. C., F. C. Zhou, S. M. Yoo, J. P. Xie, P. J. Browning, and S. J. Gao. 2004. Disruption of Kaposi's sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. J. Virol. 78:11121-11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou, J., C. Chau, Z. Deng, W. Stedman, and P. M. Lieberman. 2005. Epigenetic control of replication origins. Cell Cycle 4:889-892. [DOI] [PubMed] [Google Scholar]