Abstract
The US3 protein kinase of herpes simplex virus 1 blocks apoptosis induced by replication-incompetent virus mutants, proapoptotic members of the Bcl-2 family of proteins, and by a variety of other agents that act at the premitochondrial level in the proapoptotic cascade. To define the role of US3 in blocking apoptosis at the postmitochondrial level, we investigated the US3 protein kinase in transduced cells that were either transfected with a plasmid encoding procaspase 3 or superinfected with a proapoptotic mutant virus lacking the gene encoding the infected cell protein no. 4. (i) We show that US3 blocks the proteolytic cleavage that generates active caspase 3 from the transfected zymogen procaspase 3, concomitant with inhibition of apoptosis. (ii) Studies based on detection of fluorescence emitted upon cleavage of a synthetic caspase 3 substrate showed that expression of the US3 kinase and appearance of the cleaved substrate were mutually exclusive. (iii) An affinity-purified glutathione S-transferase (GST)-US3 fusion protein, but not the inactive GST-US3(K220N) protein, phosphorylated procaspase 3 in vitro. The studies published earlier on the effect of US3 on the upstream regulatory proteins and current studies suggest that the US3 protein kinase may act on several proteins in the proapoptotic cascade to enable the virus to complete its replication.
The studies described in this report rest on three observations. Foremost, at least two replication-defective herpes simplex virus (HSV) mutants have been shown to activate a chain of events leading to apoptosis in the infected cells. The two mutants lack the ability to express functional infected-cell protein 4 (ICP4) or ICP27 (2, 23). Wild-type virus does not induce apoptosis, although overexpression of Bcl-2 blocks or delays the appearance of cytopathic effects (10, 19).
The second fundamental observation is that wild-type virus blocks apoptosis induced by a vast variety of proapoptotic stimuli. These include a wide range of drugs or physical conditions (9, 11, 17, 20, 23, 27). Included in the list are sorbitol, an agent capable of inducing osmotic shock, and hyperthermia.
Lastly, at least four viral genes have been reported to block apoptosis. Of these, UL39, the large subunit of ribonucleotide reductase, blocks apoptosis by complementing corresponding deletion mutants only (29). Glycoprotein D appears to act by blocking massive lysosomal discharges following endocytic entry or egress (43). Glycoprotein J (17, 18, 43) has also been demonstrated to block apoptosis, although the mechanism has remained unclear. Finally, the US3 protein kinase has been shown to block apoptosis induced by a ΔICP4 virus mutant, by sorbitol, or by overexpression of proapoptotic members of the Bcl-2 family of proteins (3, 5, 6, 18, 24, 26, 27, 28). This report centers on the role of the US3 protein kinase in blocking apoptosis induced by overexpression of the effector caspase 3. Relevant to this report are the following. The US3 locus contains two overlapping transcriptional units encoding two protein kinases (30, 33). The largest product (US3) is the 481-residue protein kinase shown to block apoptosis (3, 6, 16, 24, 26-28), to play a role in the disruption of nuclear lamina to enable the transit of capsids from nuclei to the cytoplasm (36), and to phosphorylate HDAC1 and -2, essential for transactivation of genes introduced into cells by transduction (31, 32), and other proteins. The smaller product, designated US3.5, initiates at methione 77. Its spectrum of activities appears to be similar to that of US3, except that it does not block apoptosis and is partially defective in restructuring nuclear lamina in the cell line tested (30). The substrate specificities of both US3 and US3.5 kinases are virtually identical to that of protein kinase A (R-R-X-S or R-X-X-T, where X should not be an acidic amino acid residue) (4, 30), and indeed, an antibody (Ab) directed against phosphorylated protein kinase A target sequences reacts with proteins phosphorylated by US3 or US3.5 protein kinase. The evidence suggests that protection from apoptosis is most likely the result of direct phosphorylation by US3 of a cellular protein containing a protein kinase A-like target sequence (30), inasmuch as activation of protein kinase A by forskolin blocked apoptosis in a manner similar to that of US3 protein kinase (4). However, the relevant antiapoptotic targets of the US3 protein kinase have remain elusive. The evidence that US3 can block apoptosis induced by several regulatory proteins led us to explore the possibility that US3 acts in a redundant fashion both upstream on premitochondrial regulatory factors and downstream at the level of effector caspases. For this reason, we examined the ability of US3 to block activation of caspase 3, which would lead to cell death.
The various branches of the proapoptotic pathways converge at the point of activation of effector caspases 3, 6, and 7 by caspases 8 and 9 (8, 12, 40). In particular, proteolytic cleavage of procaspase 3 (the zymogen form of caspase 3) is a central event in the apoptotic process, since it generates active caspase 3, the major effector of the cascade, responsible for implementing the cell death program (34).
Although procaspase 3 can be autoactivated by self-cleavage, most likely under nonphysiologic conditions in which the zymogen is overexpressed (13-15, 25), the activation of the enzyme is tightly regulated. Activation can be blocked by survivin or p21Cip (39, 41, 42), by phosphorylation (1), or by S-nitrosylation (38). Central to the function of caspase 3 is its role in amplifying death signals. For example, active caspase 3 can cleave the proapoptotic Bcl-2 family member BAD, and the resulting cleavage product exhibits a stronger proapoptotic activity than the full-length protein (7). The relevance of positive feedback in programmed cell death is further exemplified by recent reports that caspase 3- and 7-deficient fibroblasts were highly resistant to various apoptotic stimuli and showed defects and delays in early apoptotic events at the mitochondrial level (21, 22).
In the studies reported here, we transduced cells with baculoviruses encoding the US3 kinase driven by the human cytomegalovirus immediate-early promoter in order to express the protein kinase in all or most cells in a dose-dependent manner. We selected U2OS cells because in those cells, the baculovirus-dependent transduction of the US3 protein kinase does not require the use of inhibitors of histone deacetylases (31). The ΔICP4 mutant d120 served as a positive control, since it does not express the US3 protein kinase and the enzyme readily blocks apoptosis induced by the mutant virus (24, 26). We report that (i) the US3 protein kinase blocked the activation of procaspase 3, (ii) activated caspase 3 could not be demonstrated in cells expressing the US3 protein kinase, and (iii) the US3 protein kinase phosphorylated in vitro recombinant procaspase 3.
MATERIALS AND METHODS
Cell lines and viruses.
U2OS (human osteosarcoma) cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum.
The insect cell line Sf9 (Spodoptera frugiperda) was obtained from PharMingen (San Diego, CA) and was maintained in Grace's medium supplemented with 10% fetal bovine serum. HSV-1 strain F, a limited-passage isolate, is the prototype HSV-1 strain used in this laboratory. The HSV-1(KOS) d120 mutant, a kind gift from N. DeLuca (University of Pittsburgh, Pittsburgh, PA), lacks both copies of the α4 gene and was grown in a Vero-derived cell line (E5) expressing the α4 gene. The recombinant virus R7041, lacking the US3 gene, is described elsewhere (35).
Plasmids, antibodies, and reagents.
The pORF-Casp3 plasmid, which expresses human procaspase 3 under the control of a composite promoter consisting of the elongation factor 1 alpha core promoter combined with the 5′ untranslated region of the human T-cell leukemia virus type 1 long terminal repeat, was purchased from Invitrogen (San Diego, CA). pcDNA 3.1 was purchased from Invitrogen, Carlsbad, CA. The MTS-ICP27 plasmid and the corresponding baculovirus expressing ICP27 (G. Zhou and B. Roizman, unpublished studies) were made by a standard procedure described elsewhere (27). Rabbit Ab against caspase 3 was obtained from Cell Signaling Technology, Berwyn, CA, and used at 1:500 dilution. Rabbit Ab against PARP was obtained from Santa Cruz Biotechnology (Carlsbad, CA) and used at 1:700 dilution. Monoclonal Ab against infected cell protein no. 27 was purchased from the Goodwin Institute, Plantation, FL. Rabbit Ab against US3 was described elsewhere (26).
The irreversible caspase 3 inhibitor Z-DEVD-fluoromethylketone (Z-DEVD-fmk) was purchased from Calbiochem (La Jolla, CA). Purified recombinant human procaspase 3 and human histone H1 were purchased from Calbiochem and Roche (Indianapolis, IN), respectively. The NucView reagent was purchased from Biotium, Inc. (Hayward, CA).
Baculoviruses.
Construction of control “empty” baculovirus (MTS-BAC) and US3-expressing BAC (US3-BAC) was described previously (27). US3(K220N)-BAC was made in parallel with US3-BAC; it expresses the enzymatically inactive full-length US3 protein (J. Munger and B. Roizman, unpublished studies). Recombinant baculoviruses were generated using the PharMingen baculovirus expression system as described previously. Briefly, plasmid DNA containing wild-type or mutant US3 coding sequence cloned into the baculovirus transfer vector pAc-CMV was cotransfected into Sf9 insect cells, together with the BaculoGold baculovirus DNA (PharMingen), according to the manufacturer's instructions. Supernatant containing the recombinant virus was collected and cleared by centrifugation at 2,500 rpm for 10 min 4 to 6 days after transfection, and the virus was amplified in Sf9 cells grown in a 150-cm2 flask.
Transfection and transduction protocol.
Replicate cultures of U2OS cells in 25-cm2 flasks were exposed to approximately 10 PFU of the indicated baculoviruses per cell. At 5 h after transduction, the cells were transfected with 2 μg of pORF-Casp3 plasmid. Alternatively, 2 μg of empty pcDNA or MTS-1-ICP27 plasmids was used as a transfection efficiency control. The cells were subsequently maintained at 37°C for 24 h and then at 34°C for an additional 16 h in order to avoid overgrowth.
Immunoblot assays.
Cells were harvested at the indicated times after treatment, rinsed three times with phosphate-buffered saline (PBS), and solubilized in radioimmunoprecipitation assay buffer in the presence of phosphatase inhibitors (10 mM NaF, 10 mM β-glycerophosphate, 0.1 mM sodium vanadate) and protease inhibitors (Complete; Roche). The lysed cells were stored on ice for 10 min before centrifugation at 14,000 rpm for 10 min. The protein concentrations of the supernatant fluids were determined with the aid of a Bio-Rad protein assay. Protein samples denatured in disruption buffer (50 mM Tris, pH 7.0, 2.75% sucrose, 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate) were heated at 95°C for 5 min, electrophoretically separated in denaturing polyacrylamide gels, electrically transferred to a nitrocellulose sheet, blocked, and reacted with primary Ab, followed by appropriate secondary Ab conjugated to alkaline phosphatase (Bio-Rad, Hercules, CA). Protein bands were visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Denville Scientific, Metuchen, NJ).
DEVDase activity assay.
Caspase 3 activity was assayed by using a tetrapeptide conjugated to phenylnitraniline (Calbiochem). Cells were harvested at the indicated times after infection, rinsed three times with PBS, resuspended in 75 μl of lysis solution A {0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 50 mM HEPES, pH 7.4, 1 mM dithiothreitol, 0.1 mM EDTA}, held on ice for 10 min, and centrifuged at 14,000 rpm for 10 min at 4°C. Equal amounts of protein in supernatant fluids were tested for DEVDase activity according to the manufacturer's instructions.
Purification of GST-US3 protein.
Construction of the baculovirus expressing glutathione S-transferase (GST)-US3 in Sf9 insect cells has been reported elsewhere (4). Baculovirus expressing the inactive mutant US3(K220N) amino-terminally tagged with GST was constructed with the same strategy (Munger and Roizman, unpublished). Infected Sf9 cultures were harvested, rinsed twice with PBS (0.14 M NaCl, 3 mM KCl, 10 mM Na2HPO4, 1.5 mM KH2PO4), and lysed in radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate in PBS) in the presence of protease inhibitors (Complete; Roche) as recommended by the manufacturer. Lysed cells were held on ice for 10 min before brief sonication and centrifugation at 14,000 rpm for 10 min in an Eppendorf 5415 C centrifuge. The GST chimeric proteins were bound to glutathione-Sepharose beads (Amersham Biosciences, Piscataway, NJ), rinsed four times with PBS, and stored at 4°C.
Kinase assays.
GST-US3(K220N) (2.5 μg) or GST-US3 (2.5 μg) attached to glutathione-Sepharose beads was reacted with 1 μg of procaspase 3 or histone H1 in 50 μl of kinase buffer (20 mM Tris·HCl, pH 7.5, 50 mM KCl, 10 mM MgCl2, 10 mM 2-mercaptoethanol) supplemented with 100 μM ATP and 20 μCi (1 Ci = 37 GBq) of [γ-32P]ATP. The samples were reacted at 30°C for 30 min. The beads were pelleted by low-speed centrifugation, and gel-loading buffer was added to the supernatants, which were subsequently heated to 95°C for 5 min, resolved by polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and analyzed by autoradiography. Quantification of 32P phosphorylation of the substrates was done with the aid of a Molecular Dynamics Storm 860 PhosphorImager.
Immunofluorescence analysis.
U2OS cells were seeded on four-well slides, incubated with MTS-BAC or either ∼10 or ∼0.5 PFU/cell of US3-BAC, and, after 6 h, exposed to mutant virus d120. Seventeen hours after infection, the cell culture medium was replaced by fresh medium containing the NucView reagent, according to the manufacturer's instructions. After one additional hour, the cells were fixed with 4% paraformaldehyde (PFA) in PBS for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 2 min, and incubated in blocking solution (10% fetal bovine serum in PBS) for 2 h at 4°C and then with anti-US3 Ab (1:500; 1 h 30 min) and secondary anti-rabbit Ab conjugated with Texas Red fluorescent dye (1:400; 55 min). The slide cultures were then mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA). The slides were examined in a Zeiss (Thornwood, NY) confocal microscope. Digitized images of the fluorescent Ab-stained cells were taken with a Zeiss camera (AxioCam) and were acquired with software provided by Zeiss.
RESULTS
The activation of procaspase 3 is blocked the US3 protein kinase.
The objective of these experiments was to determine the effect of the US3 protein kinase on the activity of procaspase 3 in transduced cells. Three series of experiments were done to test the hypothesis that the US3 protein kinase blocks the activation of procaspase 3.
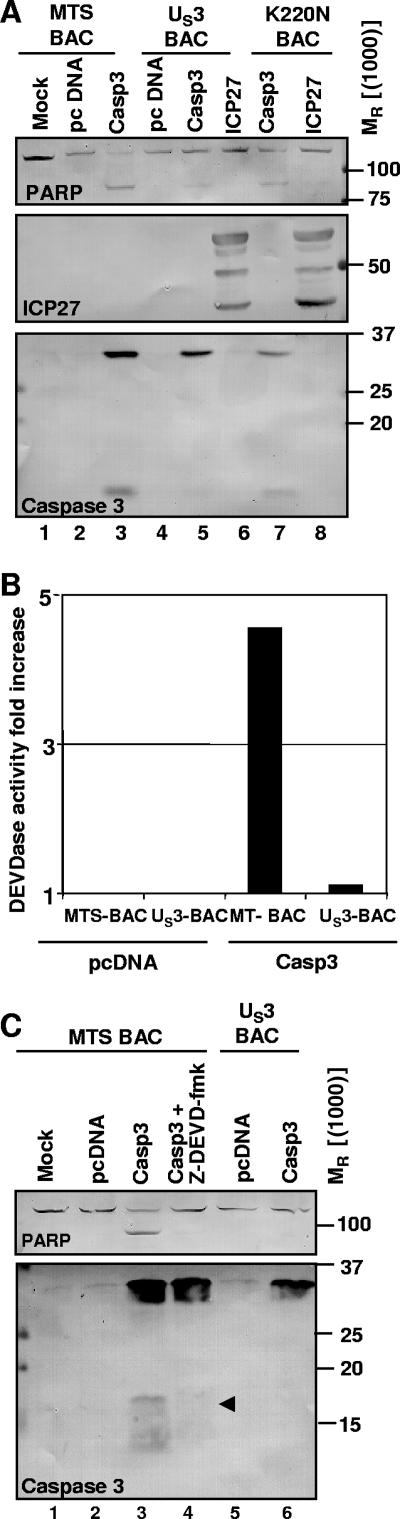
In the first series, U2OS cells were transduced with approximately 10 PFU of empty baculoviruses (MTS-Bac) or 10 PFU of recombinant baculoviruses expressing either the wild-type US3 open reading frame or the inactive kinase mutant K220N per cell. After 5 h, the cells were transfected with 2 μg of pcDNA or with the pORF-Casp3 plasmid, encoding human procaspase 3. The MTS1-ICP27 plasmid, encoding HSV-1 ICP27, was used as a control for transfection efficiency. The cells were maintained for 24 h at 37°C and for an additional 16 h at 34°C to reduce cell overgrowth. The cells were then harvested, solubilized, subjected to electrophoresis on a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against PARP, ICP27, and caspase 3, respectively. The results, shown in Fig. 1A, were as follows.
FIG. 1.
Effects of the US3 protein kinase on the processing and activity of transfected human procaspase 3. (A) U2OS cells were exposed to 10 PFU of “empty” MTS-BAC or of recombinant baculoviruses expressing either wild-type US3 or the inactive mutant US3-K220N per cell. After 5 h, the cells were transfected with 2 μg of pcDNA or the plasmid pORF-Casp3, expressing human procaspase 3. MTS-ICP27 was used as a control for transfection efficiency. The cells were maintained at 37°C for 24 h and at 34°C afterward in order to avoid overgrowth. At 40 h after transfection, the cells were harvested, solubilized, subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against PARP, ICP27, or caspase 3. (B) U2OS cells were exposed to 10 PFU of empty MTS-BAC or of recombinant baculoviruses expressing wild-type US3 per cell. After 5 h, the cells were transfected with 2 μg of pcDNA or the plasmid pORF-Casp3, expressing human procaspase 3. At 40 h after transfection, the cells were harvested, solubilized, and assayed for DEVDase activity as described in Materials and Methods. (C) U2OS cells were infected with approximately 10 PFU of empty MTS-BAC or of recombinant baculoviruses expressing wild-type US3 per cell. After 5 h, the cells were transfected with 2 μg of pcDNA or the plasmid pORF-Casp3 expressing human procaspase 3. The caspase 3 inhibitor Z-DEVD-fmk (50 μM) was added 14 h after transfection. At 40 h after transfection, the cells were harvested, solubilized, subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against PARP or caspase 3. The arrowhead points to caspase 3, the cleavage product of procaspase 3.
Procaspase 3 was cleaved in cells transduced with MTS-BAC and transfected with procaspase 3 (lane 3) and, to a lesser extent, in cells transduced with K220N and transfected with procaspase 3 (lane 7), but not in cells transduced with functional US3 and transfected with procaspase 3 (lane 5). The cleavage product had an apparent Mr of 17,000, consistent with that of the active form of the enzyme.
PARP was cleaved in cells transfected with caspase 3 in the absence of functional US3 kinase (lanes 3 and 7). The accumulating fragment had an apparent Mr of 85,000, as would be expected for the product of caspase 3-mediated cleavage.
ICP27 was expressed to the same extent in cells transduced with US3 or K220N mutant (lanes 6 and 8).
The objective of the second series of experiments was to ascertain that the procaspase 3 expressed in the absence of the US3 protein kinase was activated and expressed DEVDase activity. U2OS cells were transduced with approximately 10 PFU of MTS-BAC or of recombinant baculoviruses expressing wild-type US3 per cell. After 5 h, the cells were transfected with 2 μg of pcDNA or the plasmid pORF-Casp3 and maintained as described above. The cells were harvested 40 h after transfection, solubilized, and assayed for DEVDase activity as described in Materials and Methods. The results (Fig. 1B) show that cells transduced with MTS-BAC and transfected with procaspase 3 exhibited a marked increase in DEVDase activity compared to control cells, whereas cells transduced with US3 BAC and transfected with procaspase 3 did not.
The objectives of the third series of experiments were to further characterize the activity induced by procaspase 3. U2OS cells were transduced with approximately 10 PFU of MTS-Bac or a recombinant baculovirus expressing wild-type US3 per cell. After 5 h, the cells were transfected with 2 μg of pcDNA or the plasmid pORF-Casp3, expressing human procaspase 3. The irreversible caspase 3 inhibitor Z-DEVD-fmk (50 μM) was added 14 h after transfection. At 40 h after transfection, the cells were harvested, solubilized, subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies against PARP or caspase 3. The salient features of the results (Fig. 1C) were as follows.
Ab to caspase 3 reacted with a protein band characteristic of procaspase 3 and with a fast-migrating polypeptide in cells transduced with MTS-Bac and transfected with procaspase 3 (Fig. 1C, lane 3). This polypeptide was not detected in lysates of cells transduced with US3-Bac and transfected with procaspase 3 (lane 6) or in lysates of cells transduced with MTS-Bac, transfected with procaspase 3, and treated with the Z-DEVD-fmk inhibitor (lane 4).
We conclude from this series of experiments that in transfected cells, procaspase 3 is activated concurrently with the intracellular accumulation of the Mr 17,000 polypeptide and that the activated caspase 3 expresses DEVDase activity, cleaves PARP, and is inhibited by Z-DEVD-fmk, as would be expected of bona fide activated caspase 3. The results also show that US3 protein kinase blocks the cleavage of procaspase and its proteolytic activity.
Caspase 3 activity and US3 expression are mutually exclusive.
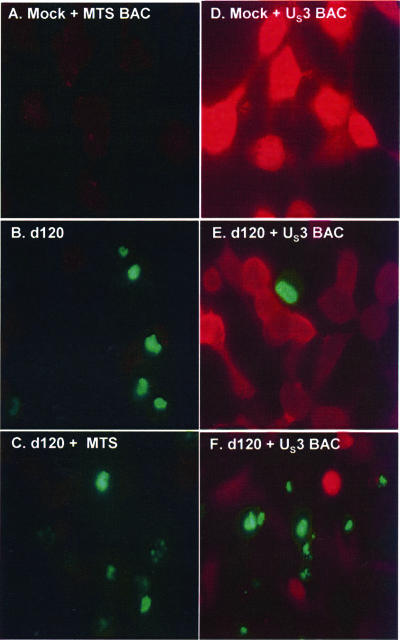
The objective of these studies was to determine whether US3 protein kinase decreases or blocks caspase 3 activation in individual cells. In this series of experiments, U2OS cells were mock treated, transduced with 10 PFU of MTS-BAC (Fig. 2A and C) or with either 10 PFU (Fig. 2D and E) or 0.5 PFU (Fig. 2F) per cell of US3-BAC. After 5 h, the cells were exposed to 10 PFU of d120 mutant virus per cell. After 17 h of incubation, the cell cultures were replenished with medium containing 5 μM NucView for 1 h. The cells were then fixed (4% PFA for 15 min), permeabilized (0.1% Triton X-100 for 2 min), and incubated in blocking solution (10% fetal bovine serum; 2 h at 4°C) and then with anti-US3 Ab (1:500; 1 h 30 min) and secondary anti-rabbit Ab conjugated with Texas Red fluorescent dye (1:400; 55 min). The NucView reagent is inert and cytoplasmic; it contains a DEVD sequence that is a target of active caspase 3. The product of such cleavage is a green fluorescent DNA dye that stains the nuclei of apoptotic cells. The cultures were examined, and images were acquired with the aid of a Zeiss confocal microscope. In all, for each experimental point, a total of 363 to 413 cells in adjacent fields were counted, in addition to a thorough examination of the cultures. The results (Fig. 2 and 3) were as follows.
FIG. 2.
Immune fluorescence analysis of U2OS cells transduced with MTS- or US3-BAC and infected with d120 mutant virus. U2OS cells were transduced with 0.5 PFU of US3-BAC (F) per cell or 10 PFU of empty MTS (A and C) or US3-BAC (E) per cell. After 5 h, the cultures were exposed to 10 PFU of mutant d120 virus per cell and maintained for 17 h. At that time, the cells were incubated in fresh medium containing 5 μM NucView for 1 h and then fixed (4% PFA for 15 min), permeabilized (0.1% Triton X-100 for 2 min), and reacted with blocking solution (10% fetal bovine serum for 2 h at 4°C) and then with anti US3 Ab (1:500; 1 h 30 min) and secondary anti-rabbit Ab conjugated with Texas Red fluorescent dye (1:400; 55 min).
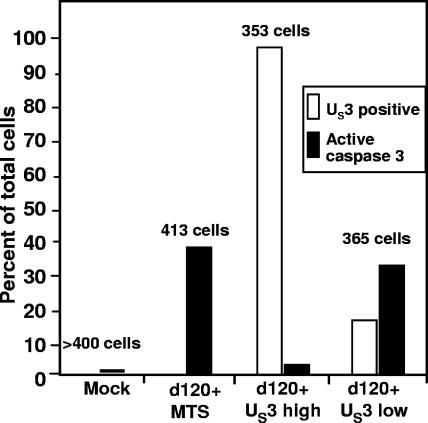
FIG. 3.
Summary of the immunofluorescence analyses of U2OS cells transduced with empty MTS or US3-BAC and infected with d120 mutant virus. The procedures are described in the legend to Fig. 2. The numbers above the bars indicate the numbers of cells counted in adjacent fields.
Examination of the mock-treated cultures (Fig. 2A) yielded 1 cell spontaneously undergoing apoptosis among an excess of 400 cells. Cultures infected with d120 mutant virus only or transduced with MTS-Bac and infected with d120 mutant virus exhibited green fluorescence indicative of active caspase 3 in 37% to 39% of the cells. In cells transduced with 10 PFU of US3-Bac per cell, 98% of cells expressed US3 protein kinase. In these cultures, the fraction of cells exhibiting active caspase 3 dropped to 1.7%. Among the cells counted, only one exhibited both US3 and active caspase 3 activities. One possible explanation for the paucity of cells exhibiting both active caspase and US3 protein kinase is that the multiplicity of US3-Bac per cell (10) was too high. In cultures exposed to 0.5 PFU of US3-Bac and 10 PFU of d120 mutant virus per cell, we found that 32% of the cells exhibited active caspase 3 and 17% of the cells exhibited US3 protein kinase. Again, only 1 cell among the 365 cells counted exhibited both active caspase 3 and US3 protein kinase. We conclude from these studies that US3 protein kinase blocks activation of procaspase 3.
The US3 protein kinase phosphorylates procaspase 3 in vitro.
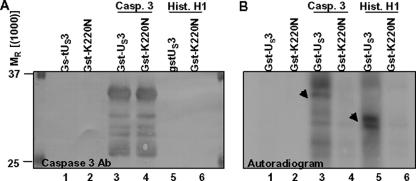
One microgram of recombinant human procaspase 3 (Fig. 4, lanes 3 and 4) or of purified histone H1 (lanes 5 to 6) was reacted with 2.5 μg of purified GST-US3 or GST-US3 K220N attached to glutathione-Sepharose beads. After a 30-min incubation at 30°C, the reaction mixtures were electrophoretically separated on a denaturing polyacrylamide gel, transferred to a nitrocellulose membrane, and either reacted with Ab to caspase 3 (Fig. 4A) or subjected to autoradiography (Fig. 4B). The results were as follows.
FIG. 4.
The US3 protein kinase can in vitro phosphorylate procaspase 3. One microgram of recombinant human procaspase 3 (Casp. 3; lanes 3 and 4) or of purified histone H1 (Hist. H1; lanes 5 and 6) was incubated with 2.5 μg of purified GST-US3 or GST-US3 K220N attached to glutathione-Sepharose beads. After a 30-min incubation at 30°C, samples were subjected to polyacrylamide gel electrophoresis, nitrocellulose membrane transfer, and either immunoblotting with anti-caspase 3 Ab (A) or autoradiography (B). Procaspase 3 protein and histone H1 protein are identified by the arrowheads.
The purified caspase 3 formed several bands that reacted with the anti-caspase 3 Ab in reaction mixtures containing wild-type US3 protein kinase (Fig. 4B, lane 3) or the inactive K220N mutant kinase (lane 4). The faster-migrating bands reactive with the anti-caspase 3 Ab more likely represented degradation products copurified with the full-length protein. Most of the bands in the reaction mixture containing active US3 protein kinase were phosphorylated (Fig. 4B, lane 3). In contrast, there was no evidence of phosphorylation of the caspase 3 in mixtures containing US3(K220N) mutated protein. It is noteworthy that a small fraction of the phosphorylated procaspase 3 protein migrated more slowly than the slowest-migrating band in Fig. 4B, lane 3. Finally, histone H1 was phosphorylated in mixtures containing the active kinase (Fig. 4B, lane 5), but not in mixtures containing the K220N protein (Fig. 4B, lane 6).
These results indicate that, at least in in vitro studies, active US3 kinase, but not the inactive US3 protein prepared under identical conditions, phosphorylated procaspase 3.
DISCUSSION
The fundamental finding that the US3 kinase blocks apoptosis emerged from studies of the proapoptotic activities of the ΔICP4 mutant d120 (23). Since then, the list has been extended to include a variety of exogenous activator and proapoptotic proteins (6, 27, 28). A central question, the target of the US3 protein kinase in blocking apoptosis, remains unresolved. The impetus for the studies described here is the observation that US3 blocks the proapoptotic proteins BAD, BAX, and BID. The hypothesis that could explain these observation is that either US3 targets all of these proteins individually or it targets a downstream effector. We expected that under the nonphysiologic overexpression of the downstream effector procaspase 3, an inefficient but effective activation of caspase 3 could be attained by self-cleavage of procaspase 3, leading to programmed cell death. In effect, what we observed was a small amount of cleavage of procaspase 3 to generate the small (Mr 17,000) active caspase 3. We have also documented evidence that in addition to the cleavage of procaspase 3, the transfected cells exhibited activities attributed to and characteristic of caspase 3. Specifically, we have shown that the transfected cells exhibit DEVDase activity and that the inhibitor Z-DEVD blocks it. We have also shown that cells transduced with a baculovirus encoding the US3 protein kinase effectively block the cleavage of procaspase 3 and also block apoptosis, whereas the enzymatically defective K220N mutant failed to block both activation and the proapoptotic activity of procaspase 3. A finding of particular interest is that in the system tested in this report, expression of US3 protein kinase and cleavage of caspase 3 substrates, detected by immunofluorescence, appeared to be mutually exclusive, even under conditions of low-multiplicity transduction by the US3 baculovirus. We also showed that procaspase 3 can serve as a substrate of the US3 kinase. Taken together, the results unambiguously demonstrate that the US3 protein kinase in the absence of other HSV-1 proteins can block the activation and proapoptotic activity of caspase 3.
Notwithstanding the evidence reported here that US3 protein kinase effectively blocks an effector caspase, we cannot exclude the possibility that the US3 protein kinase targets several proteins in the cellular proapoptotic machinery. The fundamental strategy of HSV-1 as it is evolving from the studies of viral-gene function is that key host defenses are blocked by multiple gene products or by multiple functions expressed by a single protein (37). The overall function of US3 may emerge more clearly from the functional dissections of its domains.
Acknowledgments
These studies were aided by National Cancer Institute grants CA115662, CA83939, CA71933, CA78766, and CA88860.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Alvarado-Kristensson, M., F. Melander, K. Leandersson, L. Ronnstrand, C. Wernstedt, and T. Andersson. 2004. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J. Exp. Med. 199:449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benetti, L., J. Munger, and B. Roizman. 2003. The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J. Virol. 77:6567-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetti, L., and B. Roizman. 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of ΔUS3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. 80:3341-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartier, A., T. Komai, and M. G. Masucci. 2003. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosphorylation of the Bcl-2 family member Bad. Exp. Cell Res. 291:242-250. [DOI] [PubMed] [Google Scholar]
- 7.Condorelli, F., P. Salomoni, S. Cotteret, V. Cesi, S. M. Srinivasula, E. S. Alnemri, and B. Calabretta. 2001. Caspase cleavage enhances the apoptosis-inducing effects of BAD. Mol. Cell. Biol. 21:3025-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis, R. E., J.-Y. Yuan, and H. R. Horvitz. 1991. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 7:663-698. [DOI] [PubMed] [Google Scholar]
- 9.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvan, V., R. Brandimarti, J. Munger, and B. Roizman. 2000. Bcl-2 blocks a caspase-dependent pathway of apoptosis activated by herpes simplex virus 1 infection in HEp-2 cells. J. Virol. 74:1931-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvan, V., and B. Roizman. 1998. Herpes simplex virus type 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grutter, M. G. 2000. Caspases: key players in programmed cell death. Cur. Opin. Struct. Biol. 10:649-655. [DOI] [PubMed] [Google Scholar]
- 13.Earnshaw, W. C., L. M. Martins, and S. H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383-424. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes-Alnemri, T., G. Litwack, and E. S. Alnemri. 1995. Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family. Cancer Res. 55:2737-2742. [PubMed] [Google Scholar]
- 15.Fernandes-Alnemri, T., A. Takahashi, R. Armstrong, J. Krebs, L. Fritz, K. J. Tomaselli, L. Wang, Z. Yu, C. M. Croce, G. Salveson, W. C. Earnshaw, G. Litwack, and E. S. Alnemri. 1995. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res. 55:6045-6052. [PubMed] [Google Scholar]
- 16.Hata, S., A. H. Koyama, H. Shiota, A. Adachi, F. Goshima, and Y. Nishiyama. 1999. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1:601-607. [DOI] [PubMed] [Google Scholar]
- 17.Jerome, K. R., Z. Chen, R. Lang, M. R. Torres, J. Hofmeister, S. Smith, R. Fox, C. J. Froelich, and L. Corey. 2001. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 167:3928-3935. [DOI] [PubMed] [Google Scholar]
- 18.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalamvoki, M., and B. Roizman. 2007. Bcl-2 blocks accretion or depletion of stored calcium, but has no effect on the redistribution of the IP3-1 receptor mediated by glycoprotein E of herpes simplex virus 1. J. Virol. 81:6316-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama, A. H., and Y. Miwa. 1997. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71:2567-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuribayashi, K., P. A. Mayes, and W. S. El-Deiri. 2006. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol. Ther. 5:763-765. [DOI] [PubMed] [Google Scholar]
- 22.Lakhani, S. A., A. Masud, K. Kuida, G. A. Porter, Jr., C. J. Booth, W. Z. Mehal, I. Inayat, and R. A. Flavell. 2006. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:847-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leopardi, R., and B. Roizman. 1996. The herpes simplex major regulatory protein ICP4 blocks apoptosis induced by the virus or hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacCorkle, R. A., K. W. Freeman, and D. M. Spencer. 1998. Synthetic activation of caspases: artificial death switches. Proc. Natl. Acad. Sci. USA 95:3655-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 US3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212-224. [DOI] [PubMed] [Google Scholar]
- 29.Perkins, D., E. F. R. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon, A. P., L. Benetti, and B. Roizman. 2006. US3 and US3.5 protein kinases of herpes simplex virus 1 differ with respect to their functions in blocking apoptosis and in virion maturation and egress. J. Virol. 80:3752-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poon, A. P., H. Gu, and B. Roizman. 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. USA 103:9993-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon, A. P. W., Y. Liang, and B. Roizman. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671-12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon, A. P. W., and B. Roizman. 2005. Herpes simplex virus 1 ICP22 regulates the accumulation of a shorter mRNA and of a truncated US3 protein kinase that exhibits altered functions. J. Virol. 79:8470-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter, A. G., and R. U. Janicke. 1999. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6:99-104. [DOI] [PubMed] [Google Scholar]
- 35.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. The replication of herpes simplex viruses, p. 2501-2601. In D. M. Knipe, P. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott-Williams and Wilkins, New York, NY.
- 38.Rossig, L., B. Fichtlscherer, K. Breitschopf, J. Haendeler, A. M. Zeiher, A. Mulsch, and S. Dimmeler. 1999. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J. Biol. Chem. 274:6823-6826. [DOI] [PubMed] [Google Scholar]
- 39.Shin, S., B. J. Sung, Y. S. Cho, H. J. Kim, N. C. Ha, J. I. Hwang, C. W. Chung, Y. K. Jung, and B. H. Oh. 2001. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemstry 40:1117-1123. [DOI] [PubMed] [Google Scholar]
- 40.Stennicke, H. R., and G. S. Slavesen. 1998. Properties of the caspases. Biochim. Biophys. Acta 1387:17-31. [DOI] [PubMed] [Google Scholar]
- 41.Tamm, I., Y. Wang, E. Sausville, D. A. Scudiero, N. Vigna, T. Oltersdorf, and J. C. Reed. 1998. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58:5315-5320. [PubMed] [Google Scholar]
- 42.Suzuki, A., Y. Tsutomi, K. Akahane, T. Araki, and M. Miura. 1998. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 17:931-940. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]