Abstract
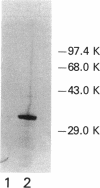
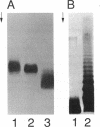
A genetically defined mutation, designated rfa-2, results in altered lipopolysaccharide (LPS) biosynthesis. rfa-2 mutants produce a core-defective LPS that contains lipid A and a single sugar moiety, 2-keto-3-deoxyoctulosonic acid, in the LPS core region. Such LPS core-defective or deep-rough (R) mutant structures were previously designated chemotype Re. Phenotypically, rfa-2 mutants exhibit increased permeability to a number of hydrophilic and hydrophobic agents. By restriction analyses and complementation studies, we clearly defined the rfa-2 gene on a 1,056-bp AluI-DraI fragment. The rfa-2 gene and the flanking rfa locus regions were completely sequenced. Additionally, the location of the rfa-2 gene on the physical map of the Escherichia coli chromosome was determined. The rfa-2 gene encodes a 36,000-dalton polypeptide in an in vivo expression system. N-terminal analysis of the purified rfa-2 gene product confirmed the first 24 amino acid residues as deduced from the nucleotide sequence of the rfa-2 gene coding region. By interspecies complementation, a Salmonella typhimurium rfaC mutant (LPS chemotype Re) is transformed with the E. coli rfa-2+ gene, and the transformant is characterized by wild-type sensitivity to novobiocin (i.e., uninhibited growth at 600 micrograms of novobiocin per ml) and restoration of the ability to synthesize wild-type LPS structures. On the basis of the identity and significant similarity of the rfa-2 gene sequence and its product to the recently defined (D. M. Sirisena, K. A. Brozek, P. R. MacLachlan, K. E. Sanderson, and C. R. H. Raetz, J. Biol. Chem. 267:18874-18884, 1992), the S. typhimurium rfaC gene sequence and its product (heptosyltransferase 1), the E. coli K-12 rfa-2 locus will be designated rfaC.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin E. A., Graves J. F., Hite L. A., Parker C. T., Schnaitman C. A. Genetic analysis of lipopolysaccharide core biosynthesis by Escherichia coli K-12: insertion mutagenesis of the rfa locus. J Bacteriol. 1990 Sep;172(9):5312–5325. doi: 10.1128/jb.172.9.5312-5325.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Sanderson K. E., Ross H. Influence of temperature on growth of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium and Salmonella minnesota. Can J Microbiol. 1976 Oct;22(10):1540–1548. doi: 10.1139/m76-226. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Biochemical construction and selection of hybrid plasmids containing specific segments of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4361–4365. doi: 10.1073/pnas.72.11.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Deshpande K. S. New cysE-pyrE-linked rfa mutation in Escherichia coli K-12 that results in a heptoseless lipopolysaccharide. J Bacteriol. 1985 Mar;161(3):1209–1214. doi: 10.1128/jb.161.3.1209-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr The rfaD gene codes for ADP-L-glycero-D-mannoheptose-6-epimerase. An enzyme required for lipopolysaccharide core biosynthesis. J Biol Chem. 1983 Feb 10;258(3):1985–1990. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Kaspar P., Zadrazil S., Fabry M. An improved double stranded DNA sequencing method using gene 32 protein. Nucleic Acids Res. 1989 May 11;17(9):3616–3616. doi: 10.1093/nar/17.9.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Biochemical characterization of mutants of Salmonella typhimurium lacking glucosyl or galactosyl lipopolysaccharide transferases. Nature. 1968 Mar 9;217(5132):957–960. doi: 10.1038/217957a0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., D'Ari L. Enzymatic incorporation of N-acetylglucosamine into cell wall lipopolysaccharide in a mutant strain of Salmonella typhimurium. Biochem Biophys Res Commun. 1964 Aug 11;16(6):568–575. doi: 10.1016/0006-291x(64)90194-9. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Pegues J. C., Chen L. S., Gordon A. W., Ding L., Coleman W. G., Jr Cloning, expression, and characterization of the Escherichia coli K-12 rfaD gene. J Bacteriol. 1990 Aug;172(8):4652–4660. doi: 10.1128/jb.172.8.4652-4660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Replacement of the fip gene of Escherichia coli by an inactive gene cloned on a plasmid. J Bacteriol. 1984 Sep;159(3):1034–1039. doi: 10.1128/jb.159.3.1034-1039.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Parker C. T., Klena J. D., Pradel E. L., Pearson N. B., Sanderson K. E., MacClachlan P. R. Physical maps of the rfa loci of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7410–7411. doi: 10.1128/jb.173.23.7410-7411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisena D. M., Brozek K. A., MacLachlan P. R., Sanderson K. E., Raetz C. R. The rfaC gene of Salmonella typhimurium. Cloning, sequencing, and enzymatic function in heptose transfer to lipopolysaccharide. J Biol Chem. 1992 Sep 15;267(26):18874–18884. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]