Abstract
A 5.9-kb DNA fragment was cloned from Pseudomonas aeruginosa PA103 by its ability to functionally complement a fur mutation in Escherichia coli. A fur null mutant E. coli strain that contains multiple copies of the 5.9-kb DNA fragment produces a 15-kDa protein which cross-reacts with a polyclonal anti-E. coli Fur serum. Sequencing of a subclone of the 5.9-kb DNA fragment identified an open reading frame predicted to encode a protein 53% identical to E. coli Fur and 49% identical to Vibrio cholerae Fur and Yersinia pestis Fur. While there is extensive homology among these Fur proteins, Fur from P. aeruginosa differs markedly at its carboxy terminus from all of the other Fur proteins. It has been proposed that this region is a metal-binding domain in E. coli Fur. A positive selection procedure involving the isolation of manganese-resistant mutants was used to isolate mutants of strain PA103 that produce altered Fur proteins. These manganese-resistant Fur mutants constitutively produce siderophores and exotoxin A when grown in concentrations of iron that normally repress their production. A multicopy plasmid carrying the P. aeruginosa fur gene restores manganese susceptibility and wild-type regulation of exotoxin A and siderophore production in these Fur mutants.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman R. A., Mildvan A. S., Loeb L. A. On the fidelity of DNA replication: manganese mutagenesis in vitro. Biochemistry. 1985 Oct 8;24(21):5810–5817. doi: 10.1021/bi00342a019. [DOI] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Jordan E. M., Wilson R. B., Draper R. K., Clowes R. C. Transcription and expression of the exotoxin A gene of Pseudomonas aeruginosa. J Gen Microbiol. 1987 Nov;133(11):3081–3091. doi: 10.1099/00221287-133-11-3081. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., HANSON J. Mutagenic action of manganese chloride. Cold Spring Harb Symp Quant Biol. 1951;16:215–228. doi: 10.1101/sqb.1951.016.01.017. [DOI] [PubMed] [Google Scholar]
- De Grandis S., Ginsberg J., Toone M., Climie S., Friesen J., Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987 Sep;169(9):4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992 Jul;174(13):4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Storey D. G., Hindahl M. S., Iglewski B. H. Differential regulation by iron of regA and toxA transcript accumulation in Pseudomonas aeruginosa. J Bacteriol. 1989 Oct;171(10):5304–5313. doi: 10.1128/jb.171.10.5304-5313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. Transcriptional regulation by iron of a Vibrio cholerae virulence gene and homology of the gene to the Escherichia coli fur system. J Bacteriol. 1990 Dec;172(12):6863–6870. doi: 10.1128/jb.172.12.6863-6870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. C., Vasil M. L. Analysis of transcription of the exotoxin A gene of Pseudomonas aeruginosa. J Bacteriol. 1986 Dec;168(3):1112–1119. doi: 10.1128/jb.168.3.1112-1119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol Gen Genet. 1984;197(2):337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Hedstrom R. C., Funk C. R., Kaper J. B., Pavlovskis O. R., Galloway D. R. Cloning of a gene involved in regulation of exotoxin A expression in Pseudomonas aeruginosa. Infect Immun. 1986 Jan;51(1):37–42. doi: 10.1128/iai.51.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H. Regulation of bacterial gene expression by metal-protein complexes. Mol Microbiol. 1990 Oct;4(10):1621–1628. doi: 10.1111/j.1365-2958.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Hindahl M. S., Frank D. W., Iglewski B. H. Molecular studies of a positive regulator of toxin A synthesis in Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:279–289. doi: 10.1159/000414353. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. The accuracy of Escherichia coli DNA polymerase I in copying natural DNA in vitro. J Biol Chem. 1980 Oct 25;255(20):9961–9966. [PubMed] [Google Scholar]
- Litwin C. M., Boyko S. A., Calderwood S. B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992 Mar;174(6):1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S. Effect of iron on accumulation of exotoxin A-specific mRNA in Pseudomonas aeruginosa. J Bacteriol. 1986 Dec;168(3):1451–1456. doi: 10.1128/jb.168.3.1451-1456.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales V. M., Bäckman A., Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991 Jan 2;97(1):39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff R. M., Vasil M. L. Identification of a new phospholipase C activity by analysis of an insertional mutation in the hemolytic phospholipase C structural gene of Pseudomonas aeruginosa. J Bacteriol. 1987 Oct;169(10):4597–4601. doi: 10.1128/jb.169.10.4597-4601.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff R. M., Wretlind B., Vasil M. L. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun. 1989 May;57(5):1369–1373. doi: 10.1128/iai.57.5.1369-1373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
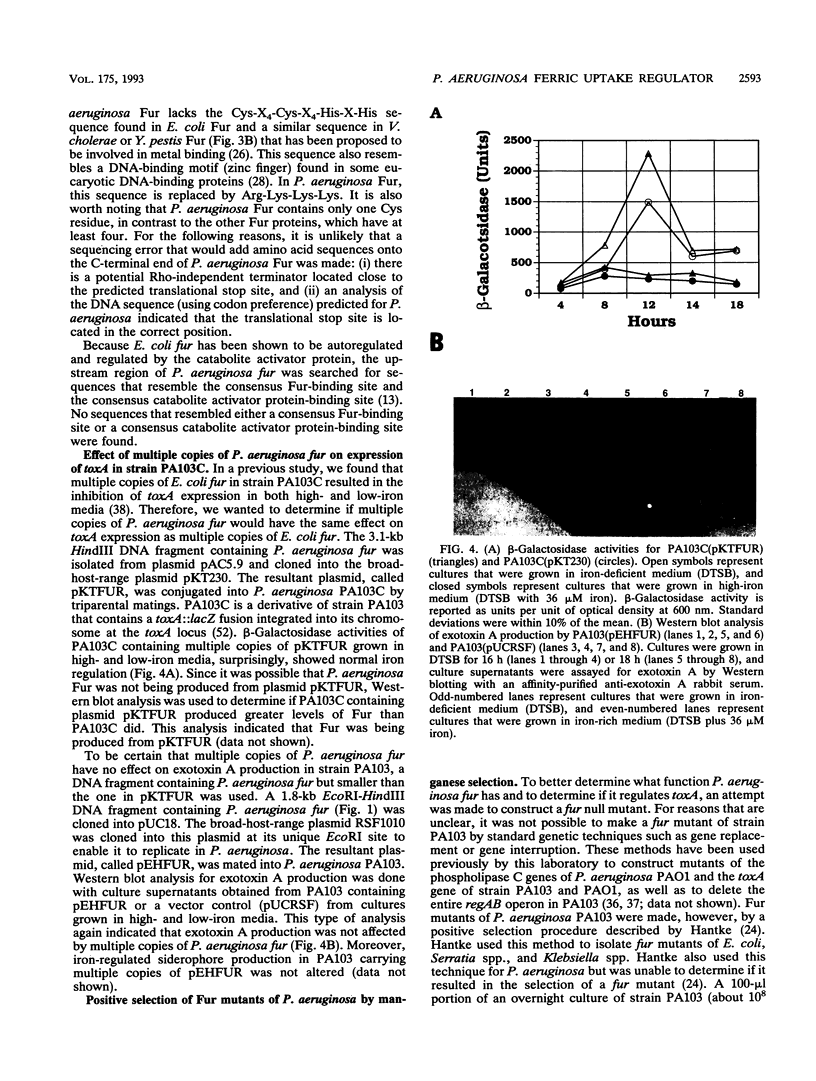
- Prince R. W., Storey D. G., Vasil A. I., Vasil M. L. Regulation of toxA and regA by the Escherichia coli fur gene and identification of a Fur homologue in Pseudomonas aeruginosa PA103 and PA01. Mol Microbiol. 1991 Nov;5(11):2823–2831. doi: 10.1111/j.1365-2958.1991.tb01991.x. [DOI] [PubMed] [Google Scholar]
- ROBERTS R. B., ALDOUS E. Manganese metabolism of Escherichia coli as related to its mutagenic action. Cold Spring Harb Symp Quant Biol. 1951;16:229–231. doi: 10.1101/sqb.1951.016.01.018. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Holmes R. K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991 Jun;59(6):1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Silver S., Johnseine P., Whitney E., Clark D. Manganese-resistant mutants of Escherichia coli: physiological and genetic studies. J Bacteriol. 1972 Apr;110(1):186–195. doi: 10.1128/jb.110.1.186-195.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Fur regulation in Yersinia species. Mol Microbiol. 1992 Sep;6(17):2507–2516. doi: 10.1111/j.1365-2958.1992.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991 Jan;173(2):417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. G., Frank D. W., Farinha M. A., Kropinski A. M., Iglewski B. H. Multiple promoters control the regulation of the Pseudomonas aeruginosa regA gene. Mol Microbiol. 1990 Mar;4(3):499–503. doi: 10.1111/j.1365-2958.1990.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Storey D. G., Raivio T. L., Frank D. W., Wick M. J., Kaye S., Iglewski B. H. Effect of regB on expression from the P1 and P2 promoters of the Pseudomonas aeruginosa regAB operon. J Bacteriol. 1991 Oct;173(19):6088–6094. doi: 10.1128/jb.173.19.6088-6094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Boyd J., Murphy J. R. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5897–5901. doi: 10.1073/pnas.89.13.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Chamberlain C., Grant C. C. Molecular studies of Pseudomonas exotoxin A gene. Infect Immun. 1986 May;52(2):538–548. doi: 10.1128/iai.52.2.538-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Grant C. C., Prince R. W. Regulation of exotoxin A synthesis in Pseudomonas aeruginosa: characterization of toxA-lacZ fusions in wild-type and mutant strains. Mol Microbiol. 1989 Mar;3(3):371–381. doi: 10.1111/j.1365-2958.1989.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Ogle J. W., Grant C. C., Vasil A. I. Recombinant DNA approaches to the study of the regulation of virulence factors and epidemiology of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:264–278. doi: 10.1159/000414352. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]






