Abstract
The cyclin-dependent kinase Cdk5 has attracted a great deal of attention both because of its roles in cell migration and axon patterning, and the extensive data implicating it in adult-onset neurodegeneration in mammals. Both the kinase activity and the biological effects of Cdk5 are absolutely dependent on association with an activating subunit, called p35. We show here that Drosophila lacking the Cdk5 activator, D-p35, display a wide range of defects in embryonic axon patterning. We further show that, while viable and fertile, p35 mutant adults display progressive, age-dependent loss of motor function and have a significantly shortened lifespan.
Introduction
Cdk5 is a member of the family of cyclin-dependent kinases, closely related to the kinases that coordinate cell cycle progression (Hellmich et al., 1992; Tsai et al., 1993). Unlike the cell-cycle kinases, however, activity of Cdk5 is largely restricted to postmitotic neurons (Hellmich et al., 1992; Tsai et al., 1993). During development, Cdk5 is essential for proper migration of CNS neurons in the mammalian cortex (Chae et al., 1997; Kwon and Tsai, 1998; Ohshima et al., 2001), and cell culture experiments implicate Cdk5 in controlling axon growth in vitro (Nikolic et al., 1996). This latter role is supported by close analysis of axon patterning in the brains of mice with reduced Cdk5 activity (Kwon et al., 1999), but the interpretation of such studies is clouded by the severe anatomical disruptions caused by defective neuronal migration in the mutants. The potential importance of Cdk5 for the development, maintenance and function of neural wiring is further indicated by the diverse group of binding partners, regulators and phosphorylation targets that have been identified for Cdk5. These include cell adhesion molecules (Cadherin (Kwon et al., 2000)), neurotransmitter receptors (NMDA receptor and associated factors (Li et al., 2001; Morabito et al., 2004)), signaling proteins (FAK (Xie et al., 2003), Abl tyrosine kinase and its cofactor, Cables (Zukerberg et al., 2000), Rac GTPase (Nikolic et al., 1998)), proteins involved in cytoskeletal structure and dynamics (tau (Baumann et al., 1993; Paudel et al., 1993), neurofilament (Lew et al., 1992), dynein associated proteins (Niethammer et al., 2000; Sasaki et al., 2000)), and many others. The presence of Cdk5 at this nexus of signaling systems, together with its phenotypic effects, makes it a very attractive candidate for playing a central integrative role in the development of neural wiring and synaptic function (Connell-Crowley et al., 2000; Xie et al., 2006).
Cdk5 has also come to prominence through a second, independent route. Aberrant post-translational modification of proteins, including phosphorylation of cytoskeletal proteins such as the microtubule-associated protein tau, is thought to play a central role in the development of human neurodegenerative diseases including Alzheimer’s Disease and Spinal Muscular Atrophy (Bancher et al., 1989; Hong et al., 1998; Iqbal et al., 2005; Paudel et al., 1993). Biochemical purification studies initially identified Cdk5 as one of the major kinases phosphorylating tau and neurofilament in mammalian neurons (Baumann et al., 1993; Lew et al., 1992; Paudel et al., 1993). Further studies showed that either increased or decreased activity of Cdk5 can lead to ultrastructural and biochemical changes associated with neurodegeneration: hyperactivation and mislocalized activation of Cdk5 leads to the hyperphosphorylated tau that is typical of several common forms of neurodegeneration (Patrick et al., 1999), and indeed can cause histological features of neurodegeneration in mouse models (Noble et al., 2003), while Cdk5-deficient mice display tangles of neurofilament and chromatolytic phenotypes typical of the degenerating nerve cells observed in spinal muscular atrophy (Ohshima et al., 1996).
Like other cyclin-dependent kinases, Cdk5 is regulated in two ways (Morgan, 1995). Its activity is absolutely dependent on binding to an activating subunit that plays the role of the cyclins in the cell cycle Cdks. In mammals, the Cdk5 activator function is provided by two closely related proteins, p35 and p39 (Lew et al., 1994; Tang et al., 1995; Tsai et al., 1994). While these protein have only limited sequence identity with cyclins, the crystal structure of a Cdk5/p35 complex reveals that p35 adopts a cyclin-like fold and stimulates Cdk5 kinase activity in a way that is similar, but not identical, to that employed by the canonical cyclins (Tarricone et al., 2001). Expression of mammalian p35 and p39 is largely neuron-specific, and it is the neuronal distribution of these regulatory subunits that accounts for the localization of Cdk5 activity to postmitotic neurons, despite the widespread expression of the Cdk catalytic subunit (Lew et al., 1995; Tang et al., 1995; Tsai et al., 1994). Cdk5 kinase activity can also be regulated by phosphorylation, particularly at amino terminal residues (Zukerberg et al., 2000), though the significance of this phosphorylation for Cdk5 function in vivo has not been established.
In order to understand the role of Cdk5 in the development of neural wiring, we previously set out to investigate the Drosophila orthologs of Cdk5 and p35 (Connell-Crowley et al., 2000). Drosophila has the advantage that there is very little cell migration in the developing embryonic CNS, removing a major confounding factor from the analysis of any axon patterning defects we observed. Studies in Drosophila are further aided by the small size, relative simplicity and stereotypic development of the fly nervous system. Moreover, only a single p35 family member has been identified in Drosophila, simplifying genetic analysis (Connell-Crowley et al., 2000). Our prior studies demonstrated that altered activity of Cdk5 produced widespread, dose-dependent defects in axon patterning. Examination of the peripheral motonerves revealed that every nerve displayed guidance errors in a fraction of hemisegments. There was, however, no simple regularity to the observed defects. Rather, in different segments the same nerve could display quite different, and even opposite phenotypes: excessive growth or stalling; failure to branch at a choicepoint or aberrant branching where it ought not to occur. Remarkably, a similar spectrum of defects was observed whether Cdk5 activity was decreased, by expression of a dominant-negative derivative, or increased, by overexpression of p35. Together, these data suggested that loss of Cdk5 activity seemed to produce a generalized deficit in the accuracy of neural wiring rather than inactivating any one, specific signaling pathway or receptor function (Connell-Crowley et al., 2000). Our ability to draw definitive conclusions from these observations, however, was impaired by the reliance of those experiments on overexpression of engineered proteins.
We have now extended our analysis of Cdk5 and p35 by generating and analyzing flies bearing null mutations in p35. We find that these mutants are viable and fertile, but display defects in axon growth and guidance that are consistent with those observed previously from expression of a dominant-negative derivative of Cdk5. Expression of the dominant negative Cdk5 in the p35 mutant background did not exacerbate the phenotype, suggesting that p35 is likely to be the major Cdk5 activator in Drosophila. Observation of adult flies mutant for p35 demonstrated an age-dependent, progressive loss of motor coordination, culminating in periods of rigidity. Finally, lack of p35 was associated with a significantly reduced adult lifespan.
Results
Isolation of null alleles of D-p35
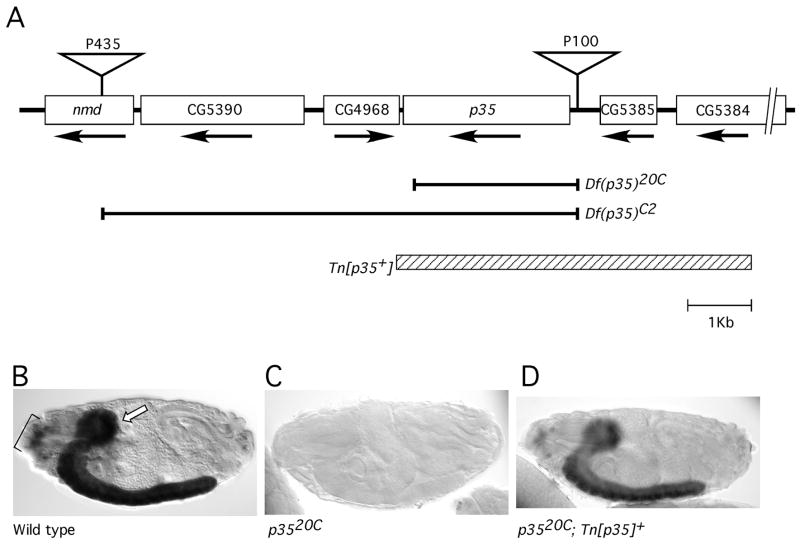
We generated two kinds of null mutations of the D-p35 gene (Fig 1A). Mobilization of a nearby P[white+] P-element (called P[435]) allowed us to generate flies with the original insertion precisely excised, but bearing a copy of the PlacW transposon between p35 and the next known transcription unit upstream of it. This insertion was identified by PCR and called P[100]. Imprecise excision of P[100] produced a series of mutations deleting the p35 promoter and different amounts of the protein coding sequence, but seemingly not affecting any other transcription unit (Figure 1). For our phenotypic analysis, we have mainly used the deletion allele p3520C, which deletes ~90% of the p35 coding region, including all sequences required for binding to and activating CDK5 (Poon et al., 1997; Tarricone et al., 2001). We also used meiotic recombination to generate a chromosome bearing both P[100] and P[435]. Introduction of P-transposase into this stock and screening for w− offspring yielded a chromosome in which both P-elements and the sequences between were deleted precisely. This stock, Df(p35)C2 is therefore null for p35 and also mutant for three adjacent genes. Analysis by in situ hybridization with a p35 probe verified the absence of detectable p35 transcript in embryos homozygous for either p3520C (Figure 1B, C) or for Df(p35)C2 (not shown). In addition to these two mutations, experiments below also make use of two large, cytologically-visible chromosomal deficiencies that uncover the p35 locus (Df(2L)J77; Df(2L)J1) (Clegg et al., 1993; Connell-Crowley et al., 2000). These deficiencies were obtained from public stock centers and are derived from a genetic background unrelated to that of the P-element-induced mutations.
Figure 1. Genomic region of p35–21C.
A. Schematic showing a ca. 12 Kb region surrounding the D-p35 gene (also called p35–21C). Predicted genes (Flybase: <ftp://flybase.org/flybase> are depicted as boxes, with the direction of transcription indicated with an arrow. Positions of P[w+] transposons discussed in the text are indicated with inverted triangles. Horizontal brackets delineate sequences that are deleted by the indicated p35 mutations; hatched box delineates genomic sequences included in a transgene (referred to as Tn[p35+]) that rescued all p35 mutant phenotypes that were analyzed.
B-D. Lateral views of embryos subjected to in situ hybridization with a p35 probe and visualized by alkaline phosphatase histochemistry. Panels display stage 17 embryos of the indicated genotypes: (B) wild type; strong labelling is observed in the CNS (arrow) and peripheral sense organs (bracket), (C) p35−, (D) p3520C; Tn[p35+]. Anterior is to the left and dorsal to the top in all embryos in this and all other figures. Apparent difference in staining intensity between Panels B (wild type) and D (rescued mutant) reflect sample-to-sample variation in histochemical staining; in parallel experiments we did not observe reproducible differences in p35 expression level between the wild type locus and the genomic transgene.
Flies lacking the p35 locus were both viable and fertile. This included homozygous p3520C flies and flies bearing the heteroallelic combinations p3520C/Df(p35)C2, p3520C/Df(2L)J77 and p3520C/Df(2L)J1. Percent viability relative to control genotypes was ~90% (average of several comparisons). We did not observe any obvious external morphological defects in mutant adults.
Widespread axon patterning defects in p35 mutant embryos
We observed a wide range of axon patterning defects in p35 null embryos. To allow comparison with previous data on Cdk5, we focused our analysis on peripheral motonerves that express the cell surface marker, Fasciclin 2 (Connell-Crowley et al., 2000). We found defects in all nerves assayed, the ISN, SNa and TN. Guidance errors included nerves that grew too far, nerves that “stalled” prematurely, axons that turned when they should not or that failed to turn when they should. Examples of some typical axonal aberrations in p35 mutant embryos are shown in Fig 2B-E. Guidance errors were observed in ~50% of embryos examined (30%–67% in different samples), but at modest expressivity, ie., only a few defects were observed per embryo, with ~20% of hemisegments showing at least one aberration in either ISN, SNa or TN. Defects were distributed rather evenly among these three nerves, though with ISN perhaps displaying somewhat less sensitivity to the mutation than the other two nerves (Fig 2F). All phenotypes we describe were seen with different imprecise excision alleles, and in different heteroallelic combinations of p35 mutations over the unrelated Df(2L)J77, demonstrating that the defects are due specifically to the lack of p35 function. Defects were not observed above background levels in embryos homozygous for the P[100] insertion that was the starting line for generation of p35 imprecise excision alleles. This modestly expressive phenotype represents the true null phenotype since the p3520C flies were maintained as a homozygous mutant stock, and embryos were therefore null for p35 both zygotically and maternally.
Figure 2. Axonal phenotypes in p35 mutant embryos.
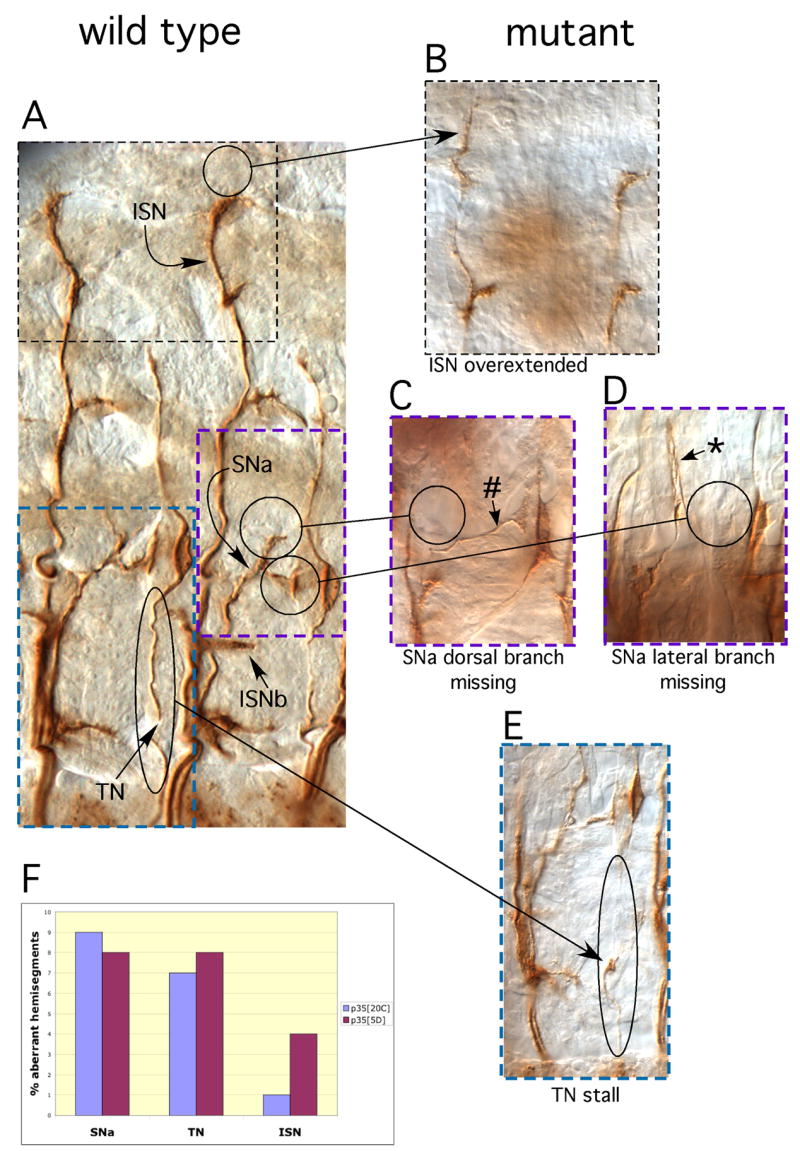
Stage 17 embryos were fixed, stained with anti-Fasciclin 2 antibodies and visualized with peroxidase histochemistry. Panel (A) shows Fasciclin 2-positive motonerves in two hemisegments of a wild type embryo; peripheral motonerves are labeled. Panels (B – E) show axonal aberrations in four different p35 mutant animals, and are typical of the range of phenotypes observed in the mutant. Each mutant panel shows a portion of a hemisegment at approximately the same scale as the wild type panel; color coded, dashed boxes on the wild type panel indicate the portion of the embryo displayed in the corresponding mutant panel. Circles and arrows highlight the defects in the mutants and the corresponding structures in the wild type; the nature of the defect is indicated below each panel. (B) Overextended ISN. (C) Missing SNa dorsal branch (lateral branch is not affected; #). (D) Missing SNa lateral branch (dorsal branch is not affected; *). E. Stalled TN. Abbreviations: ISN: intersegmental nerve, SNa: segmental nerve, “a” branch; ISNb: intersegmental nerve, “b” branch; TN: transverse nerve. (Note: TN in the more anterior segment of the wild type embryo of (C) was slightly damaged in dissection of the embryo.)
F. Histogram displaying the distribution of patterning defects among the SNa, TN and ISN peripheral motonerves for two different p35 null excision alleles, p3520C and p355D. N=180 hemisegments for each allele.
Guidance errors of a p35 mutant were non-additive with the effects of dominant-negative Cdk5
The spectrum and expressivity of axon guidance defects in the p35 mutant was very similar to the pattern of defects observed upon pan-neuronal expression of a dominant-negative Cdk5 (Connell-Crowley et al., 2000). As with Cdk5, every nerve assayed was affected at low expressivity, and all manner of guidance errors were observed. We investigated the relationship between Cdk5 and p35 by expressing the dominant negative Cdk5 in the p35 mutant background. The phenotypes of the two treatments were non-additive; motonerve defects were observed in 46% of p35 embryos, 48% of embryos expressing dominant-negative Cdk5 and 48% of p35 mutant embryos expressing dominant-negative Cdk5. Formally, this demonstrates that Cdk5 and p35 are in a common pathway; molecularly it suggests that the effect of the Cdk5 dominant negative is completely dependent on p35. Stated otherwise, these data fail to provide evidence for the presence of another Cdk5 activator protein in Drosophila.
Reduced lifespan of p35 null flies
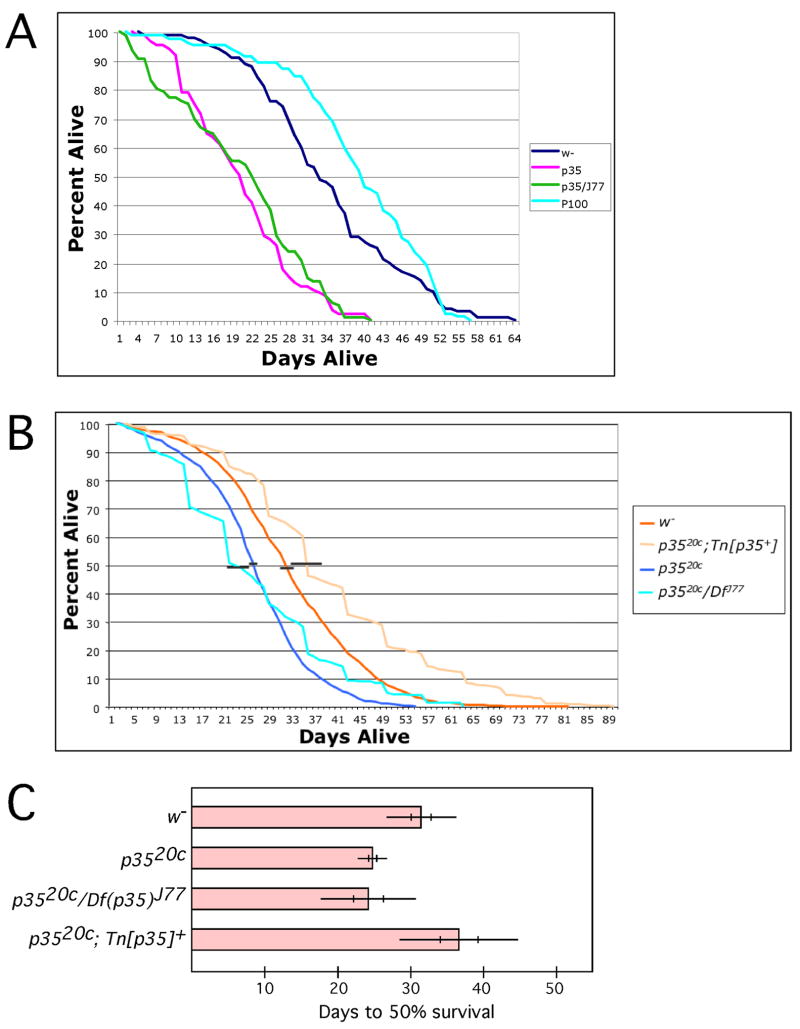
We noticed that homozygous mutant p35 stocks were difficult to maintain, with the number of live adult flies declining precipitously as a given culture aged. This led us to wonder whether the mutation affected the lifespan of the flies. We aliquoted equal numbers of newly-eclosed wild type or mutant adults into fresh vials of food, and upon allowing them to age under optimal conditions we found that the lifespan of p35− flies was reduced by about one third relative to controls. In a typical experiment (Fig 3A), 50% of wild type flies (Ore R w−) or flies bearing the P[100] P-element were still alive after 33 and 40 days respectively, with the longest-lived flies dying at 64 and 57 days. In contrast, in this experiment, the median (50%) day of death for p3520C or p3520C/Df(2L)J77 was 20 and 22 days with the last flies dying at 40 days. This decrease in lifespan by ~1/3 was highly reproducible. Figure 3B presents averaged survivorship curves derived from 13 independent experiments employing wild type (w−) or homozygous mutant (p3520c) flies, and from 10 independent experiments employing a heteroallelic combination of p35 alleles (p3520c/Df(2L)J77) or homozygous mutant bearing a rescuing transposon that carries the wild type genomic locus (p3520c; Tn[p35+]), with error bars indicating the standard error in median survival (days to 50% survival). To simplify viewing of the summary statistics, data from these same experiments is displayed in Panel C in histogram format, showing the mean time to 50% decrease in surviving population size as a bar, with standard deviations and standard errors indicated, for wild type and various mutant genotypes, as well as for homozygous mutant in the background of the rescuing genomic transposon (Tn[p35+]). Various combinations of p35 alleles all display the shortened median lifespan phenotype (25 ± 1 vs 24 ± 2 days; mean ± SEM), and it is fully rescued by a transgene bearing the p35 genomic locus (median lifespan = 36 ± 2 days). A reduction in lifespan equivalent to that seen in the p35 mutant was also produced by expression of dominant negative Cdk5 in postmitotic neurons under control of the elav-GAL4 driver (29% decrease in median lifespan, P = 0.05; paired, two-tailed T-test, N= 5 independent experiments). These data demonstrate that p35/Cdk5 function is required in post-mitotic neurons for normal lifespan.
Figure 3. Reduced adult lifespan of p35 mutant flies.
A. Flies of the indicated genotypes were maintained in parallel at 25° under optimal growth conditions. The number of surviving flies was tallied every other day and plotted as percent alive vs days after eclosion. Data from one typical experiment is shown. Df(2L)J77 is a cytologically visible deficiency that uncovers D-p35; the p35 allele used for this experiment was p3520C and P[100] is the P-element insertion that was used to generate p3520C by imprecise excision.
B. Average survivorship curves compiled from 13 independent experiments for w− and w−; p3520C, and 10 independent experiments for p3520c; Tn[p35+] and p3520c/Df(2L)J77. Each experiment was performed as described for (A), except that for some experiments employing p3520c; Tn[p35+] and p3520c/Df(2L)J77 survivorship was tallied every 5 days instead of every other day. Percent survival on a given day after eclosion was derived by averaging the fraction surviving on that day across all experiments for a given genotype. Horizontal black bars represent the standard error of the mean (SEM) for the time to 50% survival. For clarity, these bars are shown displaced slightly from the 50% level. Each experiment started with ≥100 flies of each genotype, except p35, experiment #5 (n=58) and experiment #7 (n=92).
C. Summary statistics for the data from (B). The mean number of days to 50% survival was calculated from multiple experiments for each of the indicated genotypes and is indicated by the bar. Thin horizontal lines indicate ± 1 standard deviation for each lifespan; vertical hash marks indicate ± 1 SEM. Mean lifespan of w− was not significantly different from that of p35−; Tn[p35+] and lifespan of p3520C was not significantly different from p35/Df(2L)J77, but in all pairwise comparisons, lifespan of genotypes lacking p35 was significantly different from that of p35+ genotypes (p < .05, ANOVA). Note that the “mean lifespan” reported in this panel is calculated as the mean of the medians of the separate experiments, whereas the mean survival curves in (B) are derived from compilation of all the datasets. Absolute time to “50% survival” is slightly different from these two calculations, but both support a conclusion of significant lifespan reduction in the mutant.
Adult-onset, progressive loss of motor coordination in p35 mutants
We also noticed various phenotypes suggestive of deficits in adult motor function in p35 mutant flies. Most striking were a tendency for flies to drag their legs, to fall onto their backs and be unable to right themselves, and a tendency for individuals to stand rigidly in place without moving for many seconds at a time, particularly in vials of older flies. We quantified motor function by a wall climbing assay, gently tapping the flies to the bottom of a vial and determining the fraction that climbed 5cm up the side of the vial within 10 seconds (Fig 4). We found that, at eclosion, p35 mutant flies performed the wall-climbing task nearly as well as wild type flies, with 80–90% successful, vs 90–95% for wild type. The performance of the mutants declined rapidly with age, however, with 50% of mutant flies unable to perform the task by 14–21 days of life. For comparison, while motor function of wild type flies also diminishes with age, the drop to 50% performance is typically not reached by wild type until ~42 days of life. Note that the decline in motor function is substantially faster than the decline in survival, suggesting that reduced motor function is a relatively early event in the progression to death. As in the lifespan experiments, the starting P[100] insert line and lines homozygous for the p35 mutation in the background of a p35+ genomic transgene (Tn[p35+]) performed indistinguishably from wild type, and flies bearing the heteroallelic combination p3520C/Df(2L)J77 behaved the same as p3520C homozygous flies.
Figure 4. Progressive loss of motor function in p35 mutant flies.
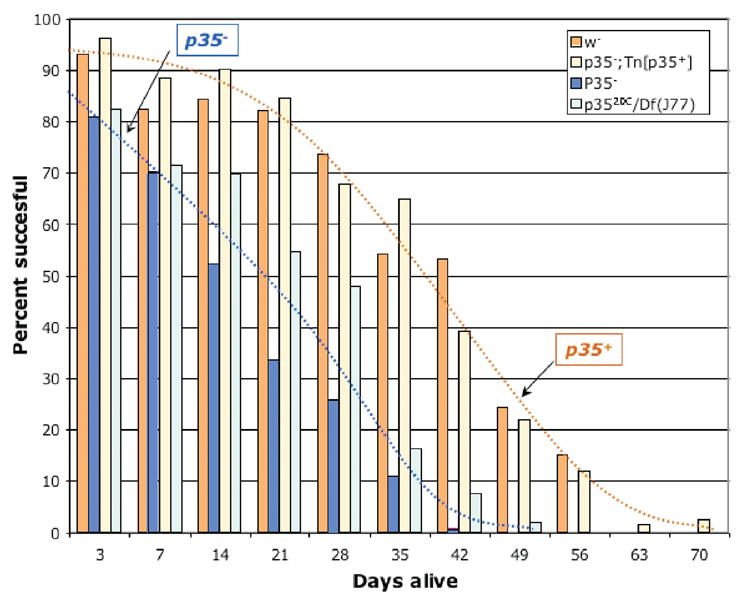
Populations of flies of the indicated genotypes were assayed weekly after eclosion for their ability to climb 5cm up the side of a vial within 10 seconds. The fraction that were successful at this task at each time point is indicated with a vertical bar. Each bar represents the average from 8 independent experiments. Standard error for each measurement displayed was ~5% (range 2–11%). Df(2L)J77 is a cytologically visible deficiency that uncovers p35, p35−; Tn[p35+] is a null mutant bearing the wild type genomic locus as a transgene, and the p35 excision allele used for this experiment was p3520C and is in a w− genetic background. Dotted lines represent approximate interpolations of the data for the two p35+ genotypes (orange), and for the two p35− genotypes (blue)
Discussion
The molecular activities, cellular pattern of expression and subcellular localization of the cyclin-dependent kinase, Cdk5, make it an excellent candidate for a molecule that controls axon patterning and synaptic function by coordinating the many signals required for the faithful establishment and maintenance of neural wiring (Connell-Crowley et al., 2000; Xie et al., 2006). Our previous studies employing overexpression of dominant transgenic constructs suggested that reduction of Cdk5 activity leads to errors in axon guidance in vivo (Connell-Crowley et al., 2000). The p35 null phenotype we report here supports those findings, confirming the necessary role of Cdk5 and p35 in embryonic axon guidance. Moreover, we find that absence of p35 causes age-dependent deficits in motor function, as demonstrated by progressive degradation of motor function, culminating in periods of rigidity, and associated with premature death.
The axon patterning defects we observe in the p35 null mutant mimic closely, both in kind and in severity, the defects produced by neuronal expression of a kinase-inactive Cdk5. This suggests that the dominant negative Cdk5 was both faithful and effective in revealing the function of Cdk5/p35. Moreover, while it is clear from the Drosophila genome sequence and from our molecular studies that there is only a single p35 family member in Drosophila, the observation that expression of the dominant-negative Cdk5 in the p35 null background does not yield a stronger phenotype than either the mutant or the transgene alone suggests that there is not likely to be another major Cdk5 activator protein of some other molecular nature in growing Drosophila axons.
Careful observation of the p35 null stock suggested the presence of behavioral deficits and reduced adult vigor. Quantitative behavioral assays supported this impression, revealing that while motor function in the mutants was nearly wild type at eclosion, it declined swiftly with advancing adult age, to be followed by periods of unmoving rigidity and then by premature death. The cellular basis of these phenotypes remains unclear. The neuron-specific pattern of p35 expression, however, and the ability to mimic the lifespan phenotype of p35 null mutants by specific expression of dominant negative Cdk5 in postmitotic neurons demonstrate that it is the function of p35 in mature, postmitotic neurons that is essential for normal lifespan. Experiments are currently in progress to test whether the lifespan and behavioral phenotypes of p35 are a delayed, secondary consequence of developmental miswiring, or whether they reflect a required adult function of this protein.
It is noteworthy that one of the obvious behavioral defects in p35 mutant adults is a tendency of the adult flies to lie on their backs, unable to right themselves. This phenomenon has been observed previously in the Mediteranean fruit fly, Ceratitis capitata, and has been termed the “supine” phenotype (Papadopoulos et al., 2002). In that system, as well, the behavior was associated with reduced lifespan and it was further shown that assumption of the supine phenotype by a particular individual was an early marker of approaching death.
The pattern of behavioral phenotypes we observe in p35 null adults bear intriguing parallels with observable features of mammalian neurodegenerative diseases, some of which have been linked to altered activity of mammalian Cdk5 (Monaco, 2004). Does neurodegeneration play a role in the progressive phenotype of p35 null mutants? Can the wild type functions and mutant phenotypes of p35 and Cdk5 in Drosophila shed any light on mammalian neurodegenerative diseases? If detailed histological and ultrastructural analysis of p35 mutants reveals anatomical degeneration with morphological similarity to mammalian neurodegeneration, it holds the promise of applying the uniquely precise tools of Drosophila genetics to investigate fundamental cellular and molecular mysteries of neurodegeneration, including the earliest events in neuropathology, cell autonomy, and the molecular genetic basis of the disease process.
Experimental Procedures
Genetic methods
P-element insertions (P[435] and P[2358]) and chromosomal deficiencies (Df(2L) J1, Df(2L)J27, Df(2L)J77 and Df(2L)J106) near the p35 locus were obtained from the Bloomington Drosophila Stock Center. UAS-transgenes for p35 and Cdk5 have been described previously (Connell-Crowley et al., 2000). Procedure for generation of small p35− transposon excisions is described in the text.
A p35+ genomic transgene was constructed by isolating a 5.5 Kb SalI –XbaI restriction fragment spanning the p35 locus from a genomic phage clone (isolated by plaque hybridization from a λDASH library), and subcloning it into the transformation vector pW8. The genomic transgene was introduced into Ore R w1118 flies by P-element mediated transformation. This transgene is referred to in the text as Tn[p35+].
Lifespan and Behavior Assays
To quantify lifespan, 20 newly-eclosed flies were aliquoted into each of 5 separate vials. Flies were maintained at 25°, and transferred to fresh vials every two days, counting the number of dead flies at each transfer, until all flies were gone. Males and females were not separated.
To quantify wall-climbing behavior, 20 newly eclosed flies (males + females) were aliquoted into each of 6 vials. At day 3 after eclosion, day 7, and then every 7th day thereafter until the flies died, wall climbing was assayed. Flies were tapped gently to the bottom of the vial, and the number that climbed up to or beyond a line 5 cm from the top of the food after 10 seconds was counted. Assay was performed three times for each vial on its testing day. The total number of live flies per vial at the time of testing was recorded, and the fraction of flies successful at the test was calculated, averaging the trials for a given sample, and then the samples for a given genotype. Median and ultimate survival times from wall-climbing experiments were in agreement with those from the lifespan assay, suggesting that the behavioral testing protocol did not seriously damage the flies.
Antibody staining and analysis of axonal phenotypes
Antibody staining, in situ hybridization, and quantification of axonal phenotypes were performed as described previously (Connell-Crowley et al., 2000).
Acknowledgments
We thank past and current members of our lab, particularly Dan Crowner, Mike Gates, Maude Le Gall, and Veta Trunova, for extensive assistance and advice during the course of these experiments, and we thank Chi-Hon Lee and Ward Odenwald for helpful comments on the manuscript and Dr. Neal Jeffries (NIH/NINDS) for advice concerning the statistical analysis of survivorship data. Fly stocks were provided by the Drosophila Genetics Stock Center, and antibodies by the Developmental Studies Hybridoma Bank. Early phases of the work were supported by NIH grant R01-GM57830 while the lab was located at the Fred Hutchinson Cancer Research Center; later phases of the work were supported by the Intramural Research Program of the NINDS, Basic Neuroscience Program, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Clegg NJ, Whitehead IP, Brock JK, Sinclair DA, Mottus R, Stromotich G, Harrington MJ, Grigliatti TA. A cytogenetic analysis of chromosomal region 31 of Drosophila melanogaster. Genetics. 1993;134:221–230. doi: 10.1093/genetics/134.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell-Crowley L, Le Gall M, Vo D, Giniger E. Cdk5 controls multiple aspects of axon patterning in vivo. Curr Biol. 2000;10:599–602. doi: 10.1016/s0960-9822(00)00487-5. [DOI] [PubMed] [Google Scholar]
- Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kwon YT, Gupta A, Zhou Y, Nikolic M, Tsai LH. Regulation of N-cadherin-mediated adhesion by the p35-Cdk5 kinase. Curr Biol. 2000;10:363–372. doi: 10.1016/s0960-9822(00)00411-5. [DOI] [PubMed] [Google Scholar]
- Kwon YT, Tsai LH. A novel disruption of cortical development in p35(−/−) mice distinct from reeler. J Comp Neurol. 1998;395:510–522. doi: 10.1002/(sici)1096-9861(19980615)395:4<510::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kwon YT, Tsai LH, Crandall JE. Callosal axon guidance defects in p35(−/−) mice. J Comp Neurol. 1999;415:218–229. doi: 10.1002/(sici)1096-9861(19991213)415:2<218::aid-cne6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Winkfein RJ, Qi Z, Aebersold R, Hunt T, Wang JH. Neuronal cdc2-like kinase is a complex of cyclin-dependent kinase 5 and a novel brain-specific regulatory subunit. Nature. 1994;371:423–425. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Lew J, Qi Z, Huang QQ, Paudel H, Matsuura I, Matsushita M, Zhu X, Wang JH. Structure, function, and regulation of neuronal Cdc2-like protein kinase. Neurobiol Aging . 1995;16:263–268. 268–270. doi: 10.1016/0197-4580(95)00014-6. [DOI] [PubMed] [Google Scholar]
- Lew J, Winkfein RJ, Paudel HK, Wang JH. Brain proline-directed kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J Biol Chem. 1992;267:25922–25926. [PubMed] [Google Scholar]
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci U S A. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco EA., 3rd Recent evidence regarding a role for Cdk5 dysregulation in Alzheimer’s disease. Curr Alzheimer Res. 2004;1:33–38. doi: 10.2174/1567205043480519. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:549–552. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, Morabito M, Tsai LH. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos Y, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ogawa M, Veeranna Hirasawa M, Longenecker G, Ishiguro K, Pant HC, Brady RO, Kulkarni AB, Mikoshiba K. Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc Natl Acad Sci U S A. 2001;98:2764–2769. doi: 10.1073/pnas.051628498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci, USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA, Muller HG, Liu X. Supine behaviour predicts the time to death in male Mediterranean fruitflies (Ceratitis capitata) Proc R Soc Lond B Biol Sci. 2002;269:1633–1637. doi: 10.1098/rspb.2002.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Paudel HK, Lew J, Ali Z, Wang JH. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer’s paired helical filaments. J Biol Chem. 1993;268:23512–23518. [PubMed] [Google Scholar]
- Poon RYC, Lew J, Hunter T. Identification of functional domains in the neuronal Cdk5 activator protein. J Biol Chem. 1997;272:5703–5708. doi: 10.1074/jbc.272.9.5703. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Tarricone C, Dhavan R, Peng J, Areces LB, Tsai LH, Musacchio A. Structure and regulation of the CDK5-p25(nck5a) complex. Mol Cell. 2001;8:657–669. doi: 10.1016/s1097-2765(01)00343-4. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VSJ, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VSJ, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- Xie Z, Samuels BA, Tsai LH. Cyclin-dependent kinase 5 permits efficient cytoskeletal remodeling--a hypothesis on neuronal migration. Cereb Cortex . 2006;16(Suppl 1):i64–68. doi: 10.1093/cercor/bhj170. [DOI] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000;26:633–646. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]