Abstract
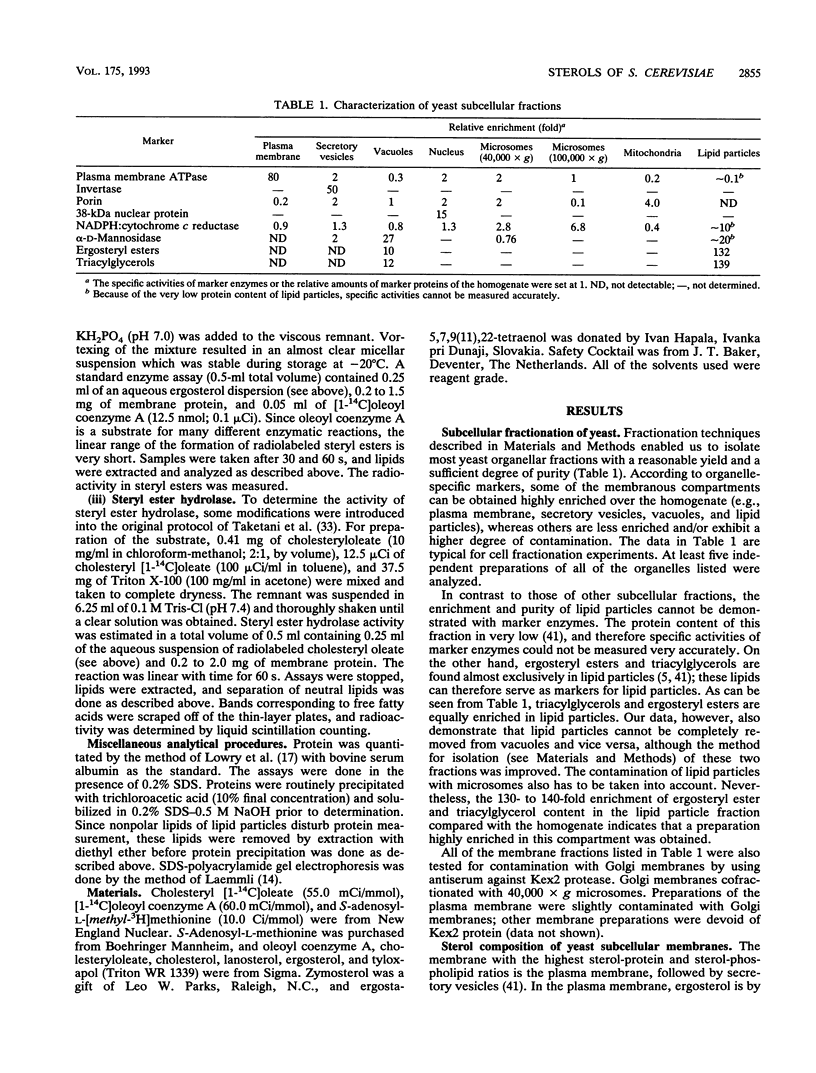
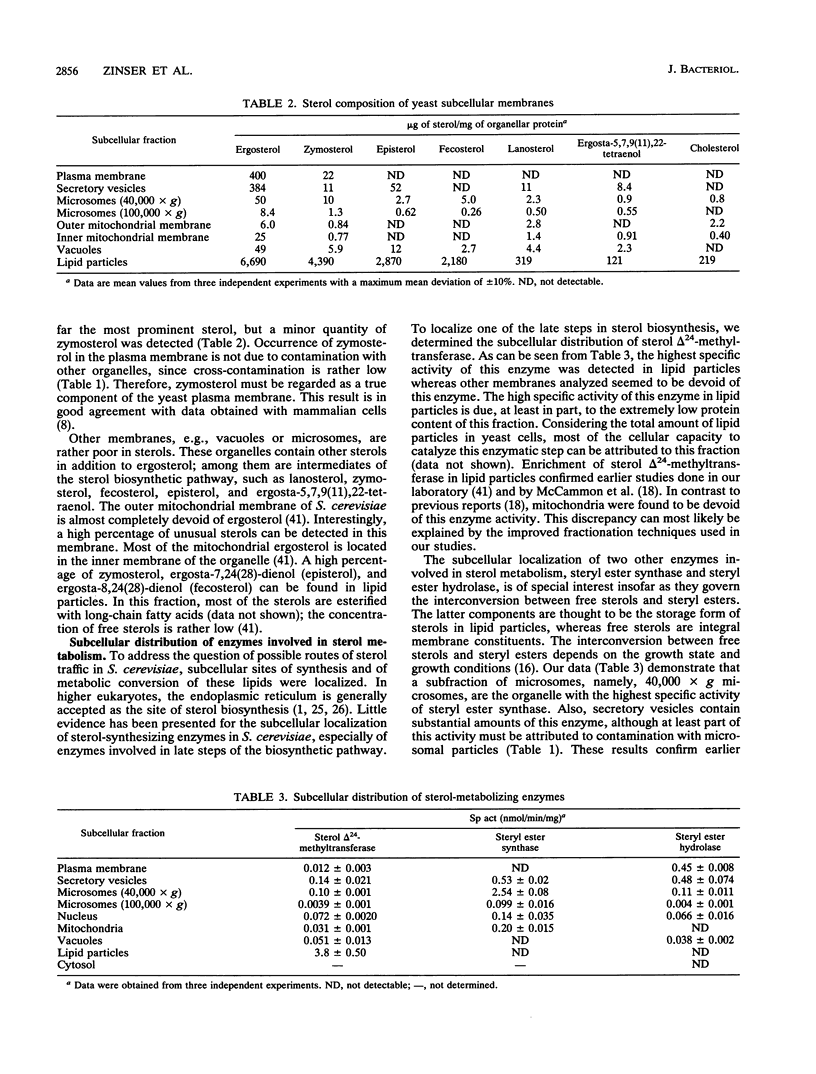
Organelles of the yeast Saccharomyces cerevisiae were isolated and analyzed for sterol composition and the activity of three enzymes involved in sterol metabolism. The plasma membrane and secretory vesicles, the fractions with the highest sterol contents, contain ergosterol as the major sterol. In other subcellular membranes, which exhibit lower sterol contents, intermediates of the sterol biosynthetic pathway were found at higher percentages. Lipid particles contain, in addition to ergosterol, large amounts of zymosterol, fecosterol, and episterol. These sterols are present esterified with long-chain fatty acids in this subcellular compartment, which also harbors practically all of the triacylglycerols present in the cell but very little phospholipids and proteins. Sterol delta 24-methyltransferase, an enzyme that catalyzes one of the late steps in sterol biosynthesis, was localized almost exclusively in lipid particles. Steryl ester formation is a microsomal process, whereas steryl ester hydrolysis occurs in the plasma membrane and in secretory vesicles. The fact that synthesis, storage, and hydrolysis of steryl esters occur in different subcellular compartments gives rise to the view that ergosteryl esters of lipid particles might serve as intermediates for the supply of ergosterol from internal membranes to the plasma membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billheimer J. T., Reinhart M. P. Intracellular trafficking of sterols. Subcell Biochem. 1990;16:301–331. doi: 10.1007/978-1-4899-1621-1_10. [DOI] [PubMed] [Google Scholar]
- Billheimer J. T., Tavani D., Nes W. R. Effect of a dispersion of cholesterol in Triton WR-1339 on acyl CoA: cholesterol acyltransferase in rat liver microsomes. Anal Biochem. 1981 Mar 1;111(2):331–335. doi: 10.1016/0003-2697(81)90570-4. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Brada D., Schekman R. Coincident localization of secretory and plasma membrane proteins in organelles of the yeast secretory pathway. J Bacteriol. 1988 Jun;170(6):2775–2783. doi: 10.1128/jb.170.6.2775-2783.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M. K., Christiansen K., Jensen P. K., Behnke O. Isolation of lipid particles from baker's yeast. FEBS Lett. 1974 Jul 15;43(2):176–179. doi: 10.1016/0014-5793(74)80994-4. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Echevarria F., Norton R. A., Nes W. D., Lange Y. Zymosterol is located in the plasma membrane of cultured human fibroblasts. J Biol Chem. 1990 May 25;265(15):8484–8489. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Holcomb C. L., Hansen W. J., Etcheverry T., Schekman R. Secretory vesicles externalize the major plasma membrane ATPase in yeast. J Cell Biol. 1988 Mar;106(3):641–648. doi: 10.1083/jcb.106.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., McDowall A., Schimmang T. Nucleolar and nuclear envelope proteins of the yeast Saccharomyces cerevisiae. Eur J Cell Biol. 1988 Aug;46(3):554–563. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange Y., Swaisgood M. H., Ramos B. V., Steck T. L. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem. 1989 Mar 5;264(7):3786–3793. [PubMed] [Google Scholar]
- Lewis T. A., Rodriguez R. J., Parks L. W. Relationship between intracellular sterol content and sterol esterification and hydrolysis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1987 Sep 25;921(2):205–212. doi: 10.1016/0005-2760(87)90020-8. [DOI] [PubMed] [Google Scholar]
- McCammon M. T., Hartmann M. A., Bottema C. D., Parks L. W. Sterol methylation in Saccharomyces cerevisiae. J Bacteriol. 1984 Feb;157(2):475–483. doi: 10.1128/jb.157.2.475-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon M. T., Parks L. W. Inhibition of sterol transmethylation by S-adenosylhomocysteine analogs. J Bacteriol. 1981 Jan;145(1):106–112. doi: 10.1128/jb.145.1.106-112.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. D., Xu S. H., Haddon W. F. Evidence for similarities and differences in the biosynthesis of fungal sterols. Steroids. 1989 Mar-May;53(3-5):533–558. doi: 10.1016/0039-128x(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Opheim D. J. alpha-D-Mannosidase of Saccharomyces cerevisiae. Characterization and modulation of activity. Biochim Biophys Acta. 1978 May 11;524(1):121–130. doi: 10.1016/0005-2744(78)90110-9. [DOI] [PubMed] [Google Scholar]
- Parks L. W., McLean-Bowen C., Taylor F. R., Hough S. Sterols in yeast subcellular fractions. Lipids. 1978 Oct;13(10):730–735. doi: 10.1007/BF02533753. [DOI] [PubMed] [Google Scholar]
- Patton J. L., Lester R. L. The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J Bacteriol. 1991 May;173(10):3101–3108. doi: 10.1128/jb.173.10.3101-3108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart M. P., Billheimer J. T., Faust J. R., Gaylor J. L. Subcellular localization of the enzymes of cholesterol biosynthesis and metabolism in rat liver. J Biol Chem. 1987 Jul 15;262(20):9649–9655. [PubMed] [Google Scholar]
- Reinhart M. P. Intracellular sterol trafficking. Experientia. 1990 Jun 15;46(6):599–611. doi: 10.1007/BF01939699. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Low C., Bottema C. D., Parks L. W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985 Dec 4;837(3):336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- SCHATZ G., KLIMA J. TRIPHOSPHOPYRIDINE NUCLEOTIDE: CYTOCHROME C REDUCTASE OF SACCHAROMYCES CEREVISIAE: A "MICROSOMAL" ENZYME. Biochim Biophys Acta. 1964 Mar 9;81:448–461. doi: 10.1016/0926-6569(64)90130-0. [DOI] [PubMed] [Google Scholar]
- Serrano R. H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- Taketani S., Nishino T., Katsuki H. Characterization of sterol-ester synthetase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1979 Oct 26;575(1):148–155. [PubMed] [Google Scholar]
- Taketani S., Osumi T., Katsuki H. Characterization of sterol-ester hydrolase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Jul 7;525(1):87–92. doi: 10.1016/0005-2744(78)90202-4. [DOI] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Metabolic interconversion of free sterols and steryl esters in Saccharomyces cerevisiae. J Bacteriol. 1978 Nov;136(2):531–537. doi: 10.1128/jb.136.2.531-537.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y. Purification of yeast vacuolar membrane H+-ATPase and enzymological discrimination of three ATP-driven proton pumps in Saccharomyces cerevisiae. Methods Enzymol. 1988;157:544–562. doi: 10.1016/0076-6879(88)57103-3. [DOI] [PubMed] [Google Scholar]
- Urbani L., Simoni R. D. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem. 1990 Feb 5;265(4):1919–1923. [PubMed] [Google Scholar]
- Walworth N. C., Novick P. J. Purification and characterization of constitutive secretory vesicles from yeast. J Cell Biol. 1987 Jul;105(1):163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. H., Norton R. A., Crumley F. G., Nes W. D. Comparison of the chromatographic properties of sterols, select additional steroids and triterpenoids: gravity-flow column liquid chromatography, thin-layer chromatography, gas-liquid chromatography and high-performance liquid chromatography. J Chromatogr. 1988 Oct 28;452:377–398. doi: 10.1016/s0021-9673(01)81462-x. [DOI] [PubMed] [Google Scholar]
- Zinser E., Sperka-Gottlieb C. D., Fasch E. V., Kohlwein S. D., Paltauf F., Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991 Mar;173(6):2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]


