Abstract
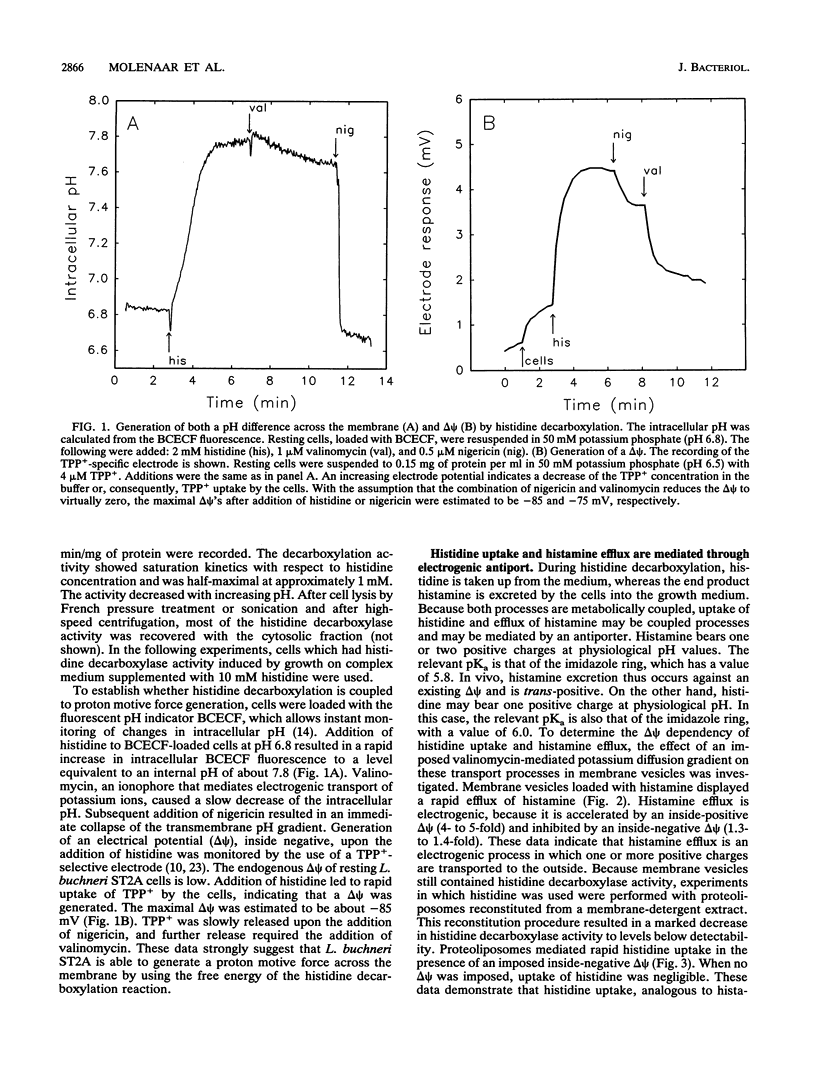
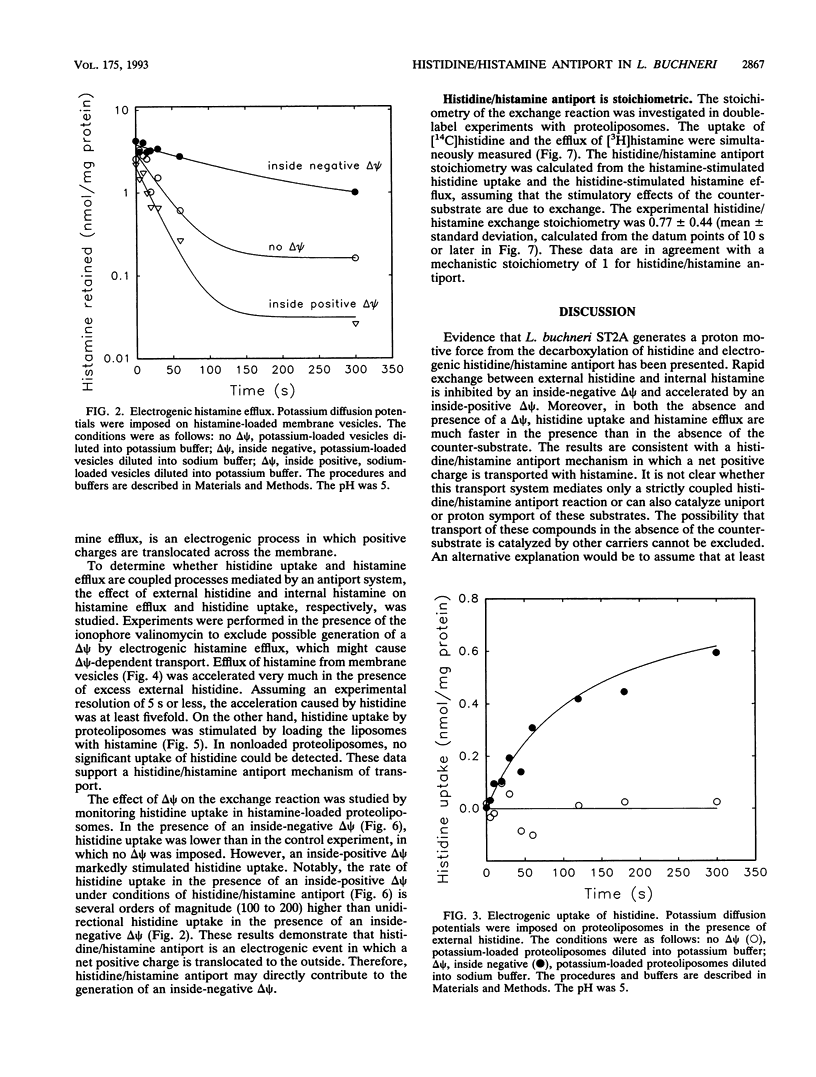
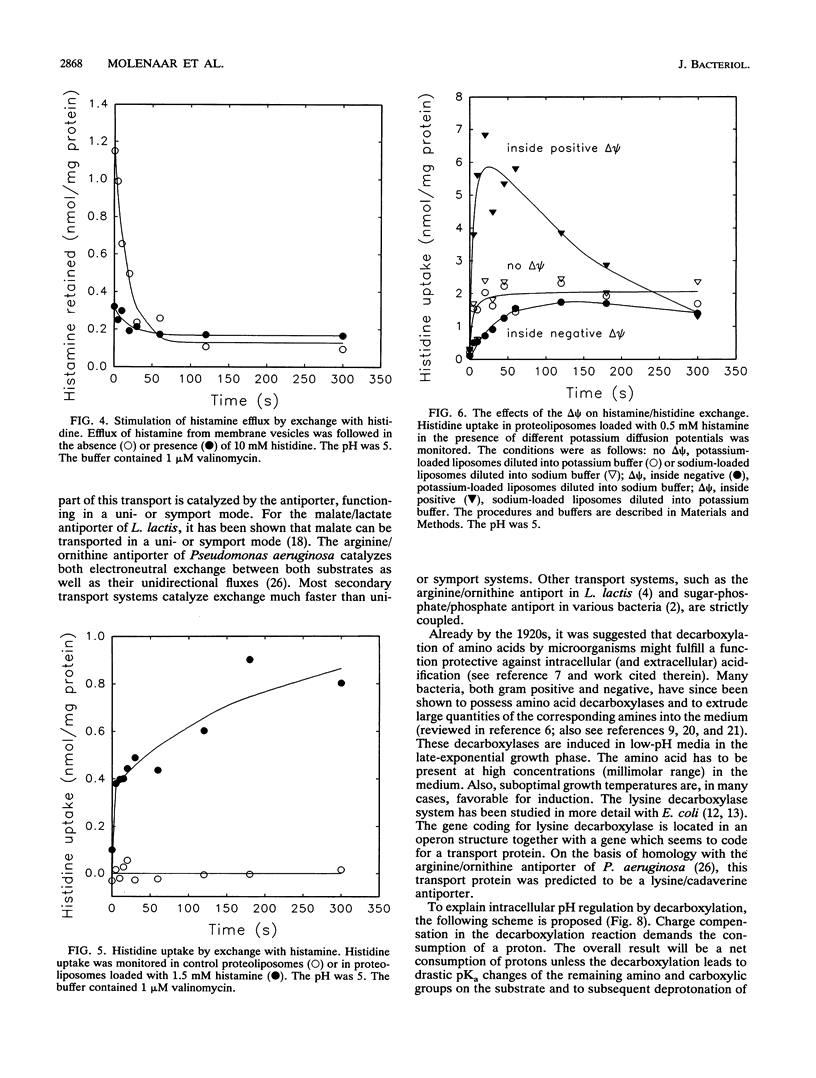
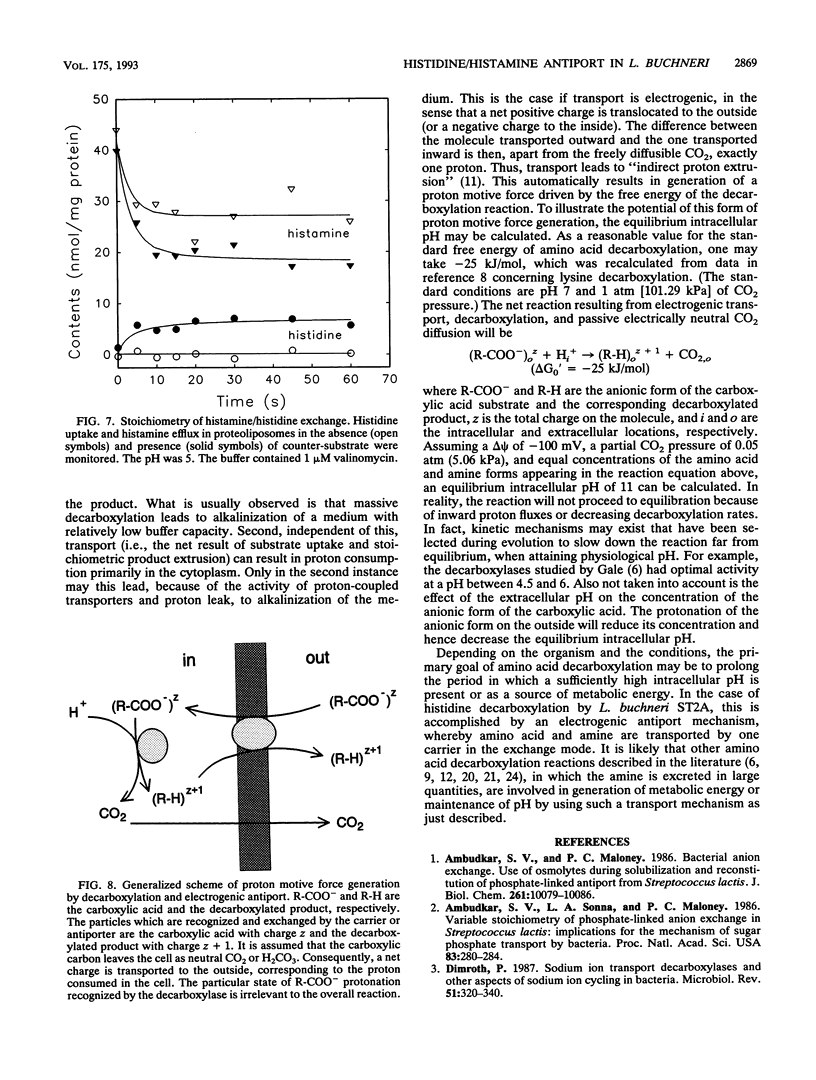
Lactobacillus buchneri ST2A vigorously decarboxylates histidine to the biogenic amine histamine, which is excreted into the medium. Cells grown in the presence of histidine generate both a transmembrane pH gradient, inside alkaline, and an electrical potential (delta psi), inside negative, upon addition of histidine. Studies of the mechanism of histidine uptake and histamine excretion in membrane vesicles and proteoliposomes devoid of cytosolic histidine decarboxylase activity demonstrate that histidine uptake, histamine efflux, and histidine/histamine exchange are electrogenic processes. Histidine/histamine exchange is much faster than the unidirectional fluxes of these substrates, is inhibited by an inside-negative delta psi and is stimulated by an inside positive delta psi. These data suggest that the generation of metabolic energy from histidine decarboxylation results from an electrogenic histidine/histamine exchange and indirect proton extrusion due to the combined action of the decarboxylase and carrier-mediated exchange. The abundance of amino acid decarboxylation reactions among bacteria suggests that this mechanism of metabolic energy generation and/or pH regulation is widespread.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Maloney P. C. Bacterial anion exchange. Use of osmolytes during solubilization and reconstitution of phosphate-linked antiport from Streptococcus lactis. J Biol Chem. 1986 Aug 5;261(22):10079–10086. [PubMed] [Google Scholar]
- Ambudkar S. V., Sonna L. A., Maloney P. C. Variable stoichiometry of phosphate-linked anion exchange in Streptococcus lactis: implications for the mechanism of sugar phosphate transport by bacteria. Proc Natl Acad Sci U S A. 1986 Jan;83(2):280–284. doi: 10.1073/pnas.83.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P. Sodium ion transport decarboxylases and other aspects of sodium ion cycling in bacteria. Microbiol Rev. 1987 Sep;51(3):320–340. doi: 10.1128/mr.51.3.320-340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Molenaar D., Konings W. N. Kinetic mechanism and specificity of the arginine-ornithine antiporter of Lactococcus lactis. J Biol Chem. 1989 Jun 25;264(18):10361–10370. [PubMed] [Google Scholar]
- Driessen A. J., Smid E. J., Konings W. N. Transport of diamines by Enterococcus faecalis is mediated by an agmatine-putrescine antiporter. J Bacteriol. 1988 Oct;170(10):4522–4527. doi: 10.1128/jb.170.10.4522-4527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPPELMAN R., MANDELES S., HANKE M. E. Use of enzymes and radiocarbon in estimation of the equilibrium constants for the decarboxylation of lysine and glutamate. J Biol Chem. 1958 Jan;230(1):73–80. [PubMed] [Google Scholar]
- LAGERBORG V. A., CLAPPER W. E. Amino acid decarboxylases of lactic acid bacteria. J Bacteriol. 1952 Mar;63(3):393–397. doi: 10.1128/jb.63.3.393-397.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Anantharam V., Allison M. J. Measurement of the substrate dissociation constant of a solubilized membrane carrier. Substrate stabilization of OxlT, the anion exchange protein of Oxalobacter formigenes. J Biol Chem. 1992 May 25;267(15):10531–10536. [PubMed] [Google Scholar]
- Meng S. Y., Bennett G. N. Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J Bacteriol. 1992 Apr;174(8):2659–2669. doi: 10.1128/jb.174.8.2659-2669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S. Y., Bennett G. N. Regulation of the Escherichia coli cad operon: location of a site required for acid induction. J Bacteriol. 1992 Apr;174(8):2670–2678. doi: 10.1128/jb.174.8.2670-2678.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar D., Abee T., Konings W. N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991 Nov 14;1115(1):75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Molenaar D., Smid E. J., Ubbink T., Abee T., Renault P. P., Konings W. N. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J Bacteriol. 1991 Oct;173(19):6030–6037. doi: 10.1128/jb.173.19.6030-6037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B. Precursor/product antiport in bacteria. Mol Microbiol. 1990 Oct;4(10):1629–1636. doi: 10.1111/j.1365-2958.1990.tb00539.x. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W. The occurrence and distribution of amino-acid decarboxylases within the genus Lactobacillus. J Gen Microbiol. 1953 Apr;8(2):224–232. doi: 10.1099/00221287-8-2-224. [DOI] [PubMed] [Google Scholar]
- Ruan Z. S., Anantharam V., Crawford I. T., Ambudkar S. V., Rhee S. Y., Allison M. J., Maloney P. C. Identification, purification, and reconstitution of OxlT, the oxalate: formate antiport protein of Oxalobacter formigenes. J Biol Chem. 1992 May 25;267(15):10537–10543. [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Sumner S. S., Speckhard M. W., Somers E. B., Taylor S. L. Isolation of histamine-producing Lactobacillus buchneri from Swiss cheese implicated in a food poisoning outbreak. Appl Environ Microbiol. 1985 Oct;50(4):1094–1096. doi: 10.1128/aem.50.4.1094-1096.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoogt H. J., Smit H., Abee T., Gamper M., Driessen A. J., Haas D., Konings W. N. arcD, the first gene of the arc operon for anaerobic arginine catabolism in Pseudomonas aeruginosa, encodes an arginine-ornithine exchanger. J Bacteriol. 1992 Mar;174(5):1568–1573. doi: 10.1128/jb.174.5.1568-1573.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- ten Brink B., Damink C., Joosten H. M., Huis in 't Veld J. H. Occurrence and formation of biologically active amines in foods. Int J Food Microbiol. 1990 Aug;11(1):73–84. doi: 10.1016/0168-1605(90)90040-c. [DOI] [PubMed] [Google Scholar]


