Abstract
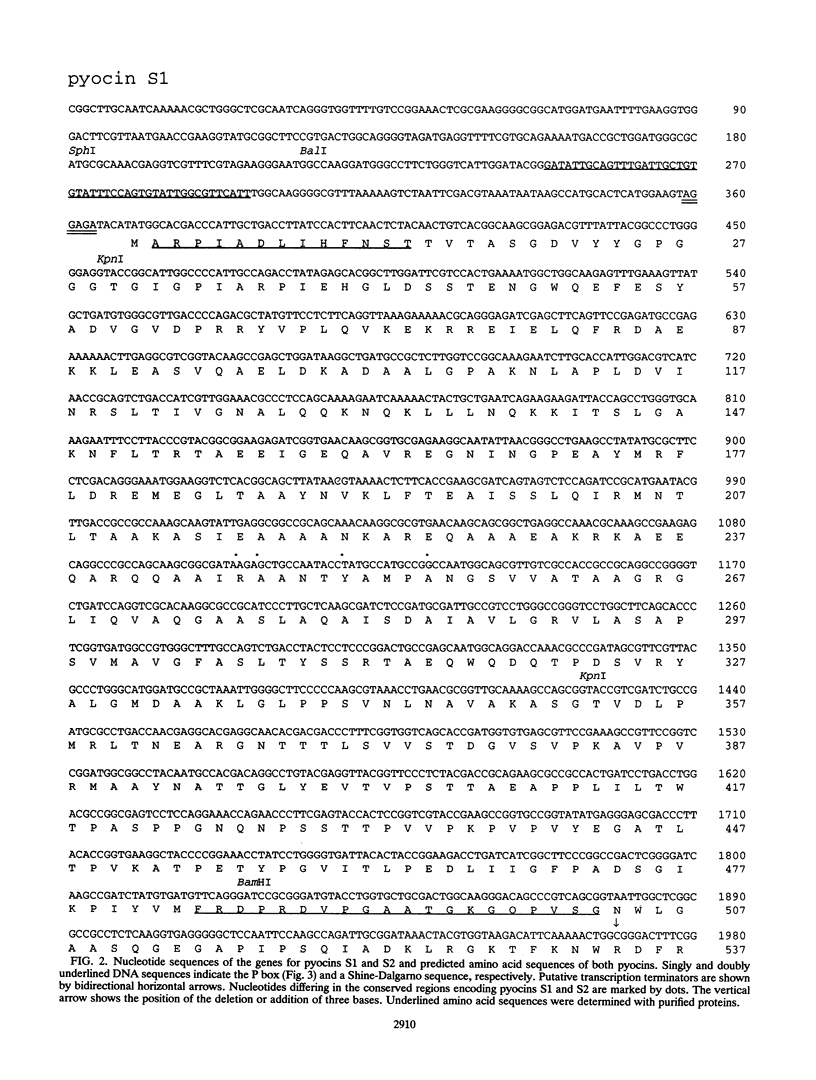
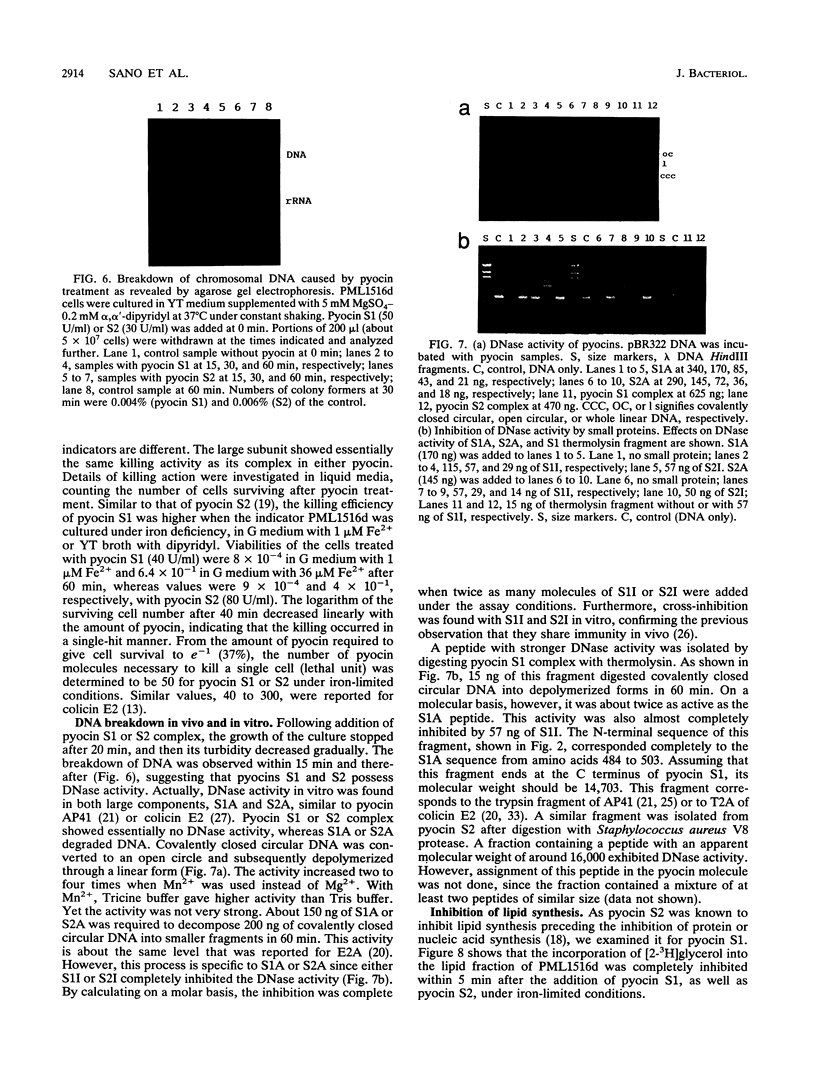
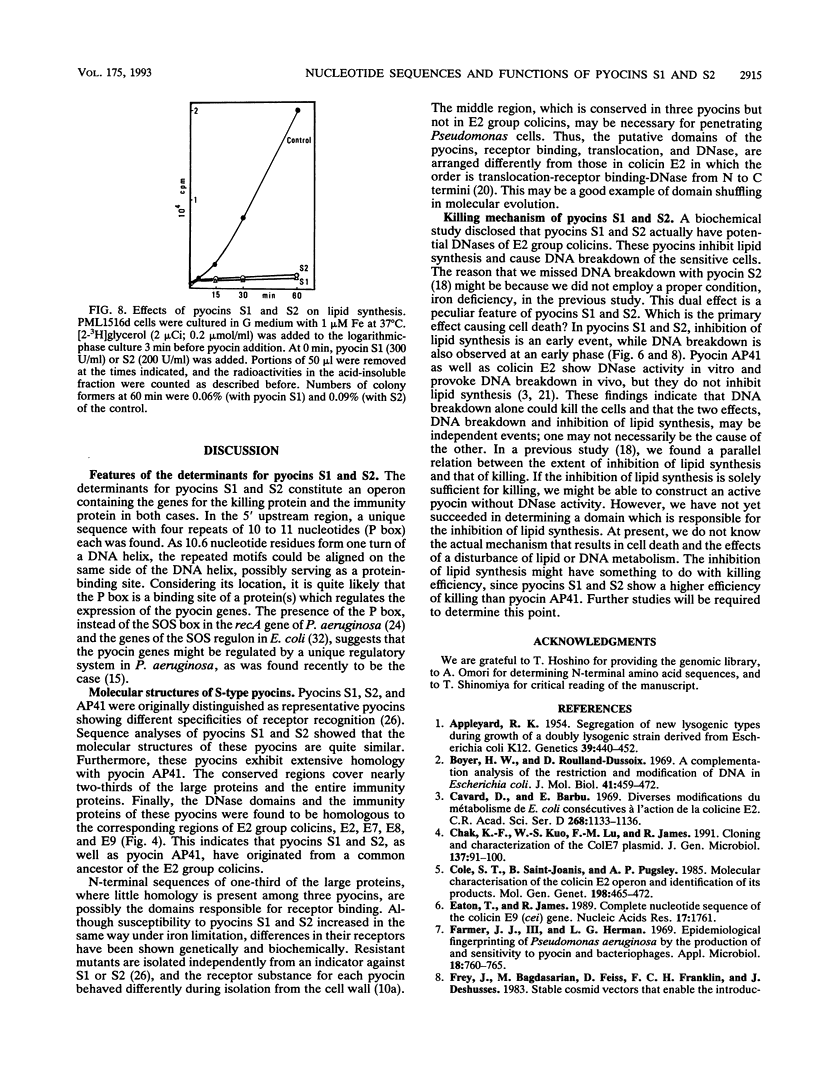
Pyocins S1 and S2 are S-type bacteriocins of Pseudomonas aeruginosa with different receptor recognition specificities. The genetic determinants of these pyocins have been cloned from the chromosomes of P. aeruginosa NIH-H and PAO, respectively. Each determinant constitutes an operon encoding two proteins of molecular weights 65,600 and 10,000 (pyocin S1) or 74,000 and 10,000 (pyocin S2) with a characteristic sequence (P box), a possible regulatory element involved in the induction of pyocin production, in the 5' upstream region. These pyocins have almost identical primary sequences; only the amino-terminal portions of the large proteins are substantially different. The sequence homology suggests that pyocins S1 and S2, like pyocin AP41, originated from a common ancestor of the E2 group colicins. Purified pyocins S1 and S2 make up a complex of the two proteins. Both pyocins cause breakdown of chromosomal DNA as well as complete inhibition of lipid synthesis in sensitive cells. The large protein, but not the pyocin complex, shows in vitro DNase activity. This activity is inhibited by the small protein of either pyocin. Putative domain structures of these pyocins and their killing mechanism are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cavard D., Barbu E. Diverses modifications du métabolisme de E. coli consécutives à l'action de la colicine E2. C R Acad Sci Hebd Seances Acad Sci D. 1969 Feb 17;268(7):1133–1136. [PubMed] [Google Scholar]
- Chak K. F., Kuo W. S., Lu F. M., James R. Cloning and characterization of the ColE7 plasmid. J Gen Microbiol. 1991 Jan;137(1):91–100. doi: 10.1099/00221287-137-1-91. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Saint-Joanis B., Pugsley A. P. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. doi: 10.1007/BF00332940. [DOI] [PubMed] [Google Scholar]
- Eaton T., James R. Complete nucleotide sequence of the colicin E9 (cei) gene. Nucleic Acids Res. 1989 Feb 25;17(4):1761–1761. doi: 10.1093/nar/17.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Herman L. G. Epidemiological fingerprinting of Pseudomonas aeruginosa by the production of and sensitivity of pyocin and bacteriophage. Appl Microbiol. 1969 Nov;18(5):760–765. doi: 10.1128/am.18.5.760-765.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- James R., Jarvis M., Barker D. F. Nucleotide sequence of the immunity and lysis region of the ColE9-J plasmid. J Gen Microbiol. 1987 Jun;133(6):1553–1562. doi: 10.1099/00221287-133-6-1553. [DOI] [PubMed] [Google Scholar]
- Lau P. C., Condie J. A. Nucleotide sequences from the colicin E5, E6 and E9 operons: presence of a degenerate transposon-like structure in the ColE9-J plasmid. Mol Gen Genet. 1989 Jun;217(2-3):269–277. doi: 10.1007/BF02464892. [DOI] [PubMed] [Google Scholar]
- Lau P. C., Rowsome R. W., Zuker M., Visentin L. P. Comparative nucleotide sequences encoding the immunity proteins and the carboxyl-terminal peptides of colicins E2 and E3. Nucleic Acids Res. 1984 Nov 26;12(22):8733–8745. doi: 10.1093/nar/12.22.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Nomura M. Interaction of colicins with bacterial cells. I. Studies with radioactive colicins. J Bacteriol. 1966 Feb;91(2):685–694. doi: 10.1128/jb.91.2.685-694.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H., Toba M., Ohta T. Structure and expression of the ColE2-P9 immunity gene. Nucleic Acids Res. 1985 Mar 11;13(5):1623–1635. doi: 10.1093/nar/13.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Sano Y., Ishihara H., Shinomiya T. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J Bacteriol. 1993 Mar;175(5):1257–1263. doi: 10.1128/jb.175.5.1257-1263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa I., Shiga S., Kageyama M. Effect of iron concentration in the growth medium on the sensitivity of Pseudomonas aeruginosa to pyocin S2. J Biochem. 1980 Jan;87(1):323–331. doi: 10.1093/oxfordjournals.jbchem.a132740. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Assignment of the functional loci in colicin E2 and E3 molecules by the characterization of their proteolytic fragments. Biochemistry. 1980 Feb 19;19(4):652–659. doi: 10.1021/bi00545a008. [DOI] [PubMed] [Google Scholar]
- Okawa I., Kageyama M., Egami F. Purification and properties of pyocin S2. J Biochem. 1973 Feb;73(2):281–289. [PubMed] [Google Scholar]
- Okawa I., Maruo B., Kageyama M. Preferential inhibition of lipid synthesis by the bacteriocin pyocin S2. J Biochem. 1975 Jul;78(1):213–223. [PubMed] [Google Scholar]
- Sano Y., Kageyama M. A novel transposon-like structure carries the genes for pyocin AP41, a Pseudomonas aeruginosa bacteriocin with a DNase domain homology to E2 group colicins. Mol Gen Genet. 1993 Feb;237(1-2):161–170. doi: 10.1007/BF00282797. [DOI] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. Genetic determinant of pyocin AP41 as an insert in the Pseudomonas aeruginosa chromosome. J Bacteriol. 1984 May;158(2):562–570. doi: 10.1128/jb.158.2.562-570.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. Purification and properties of an S-type pyocin, pyocin AP41. J Bacteriol. 1981 May;146(2):733–739. doi: 10.1128/jb.146.2.733-739.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. The sequence and function of the recA gene and its protein in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1987 Jul;208(3):412–419. doi: 10.1007/BF00328132. [DOI] [PubMed] [Google Scholar]
- Sano Y. The inherent DNase of pyocin AP41 causes breakdown of chromosomal DNA. J Bacteriol. 1993 Feb;175(3):912–915. doi: 10.1128/jb.175.3.912-915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong B. W., Lu F. M., Chak K. F. Characterization of the cea gene of the ColE7 plasmid. Mol Gen Genet. 1992 May;233(1-2):177–183. doi: 10.1007/BF00587577. [DOI] [PubMed] [Google Scholar]
- Toba M., Masaki H., Ohta T. Colicin E8, a DNase which indicates an evolutionary relationship between colicins E2 and E3. J Bacteriol. 1988 Jul;170(7):3237–3242. doi: 10.1128/jb.170.7.3237-3242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura T., Lau P. C. Nucleotide sequences from the colicin E8 operon: homology with plasmid ColE2-P9. Mol Gen Genet. 1987 Oct;209(3):489–493. doi: 10.1007/BF00331154. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Nishida K. I., Beppu T., Arima K. Tryptic digestion of colicin E2 and its active fragment. J Biochem. 1978 Mar;83(3):827–834. doi: 10.1093/oxfordjournals.jbchem.a131979. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]