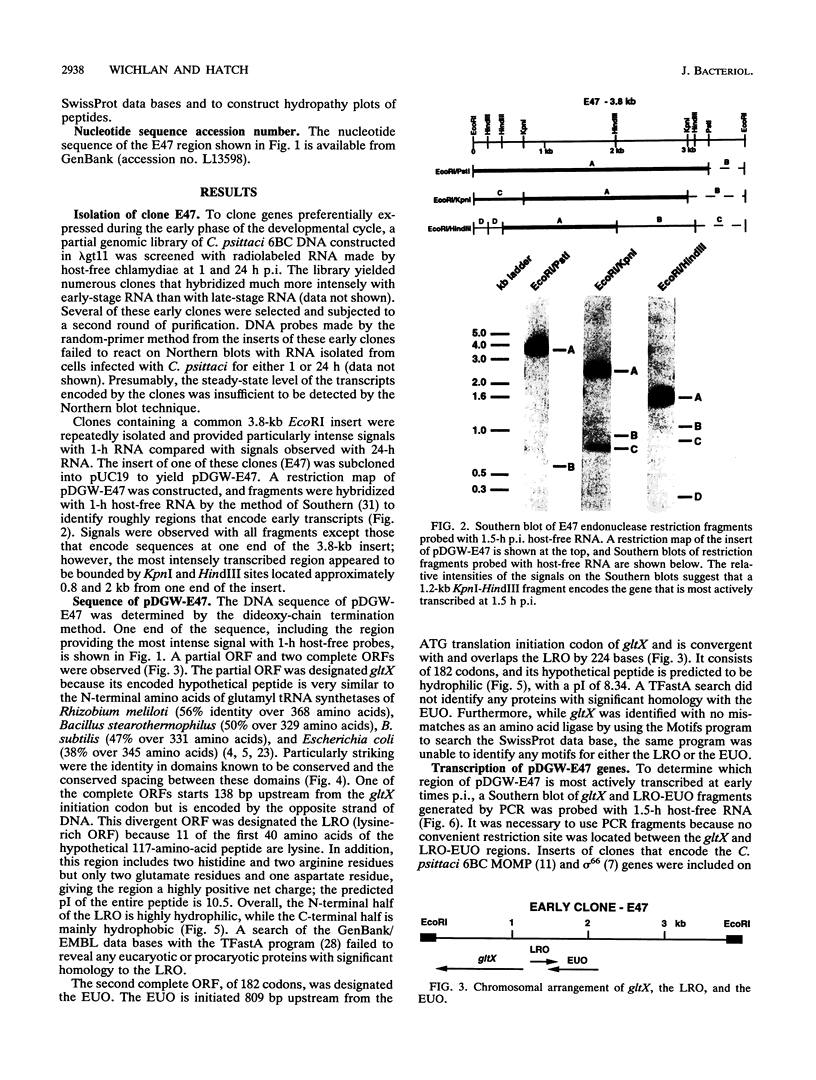
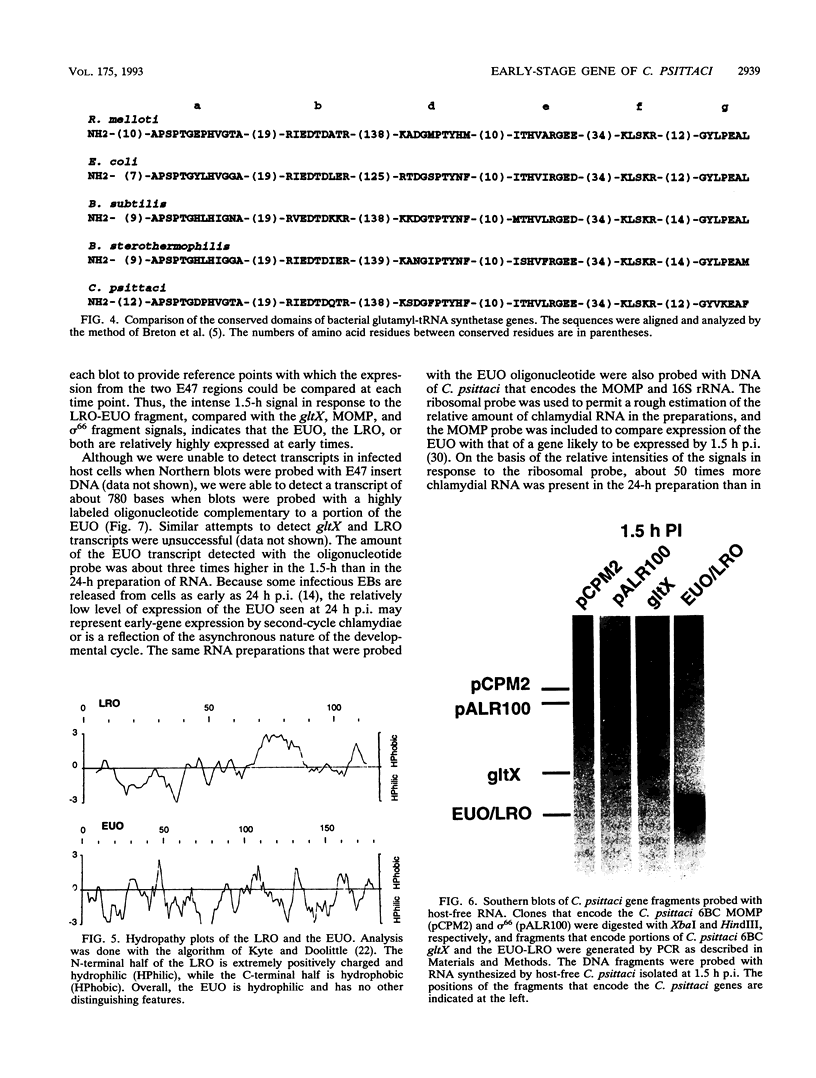
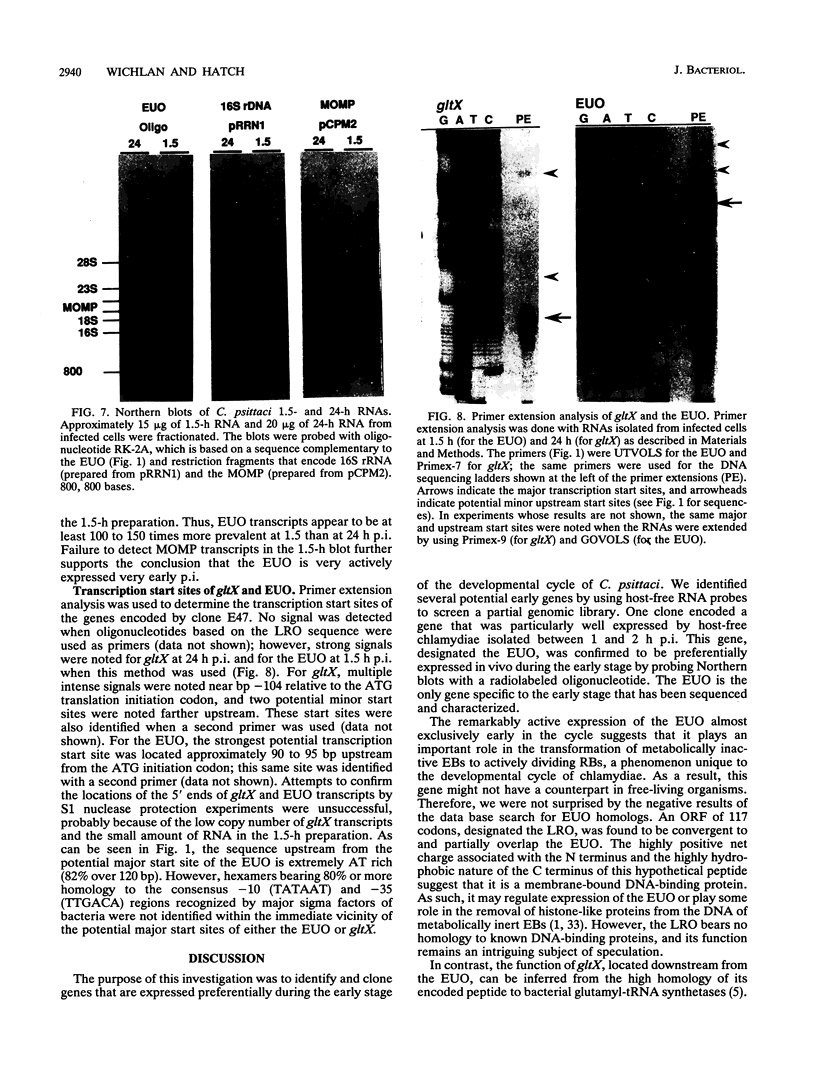
Abstract
Chlamydiae are parasitic bacteria characterized by a temporally regulated developmental cycle. In the early stage of the cycle, metabolically inert elementary bodies reorganize to dividing reticulate bodies, a process about which little is known. The purpose of this investigation was to identify and clone chlamydial genes that are expressed preferentially during the early stage of the developmental cycle of Chlamydia psittaci 6BC. Several potential early genes were cloned with highly radioactive, host-free-generated RNA probes to screen a genomic library. One clone appeared to encode a gene that was particularly well expressed at 1 h postinfection. In further characterization, we found that it encodes two complete open reading frames and one partial open reading frame of 370 codons. The partial open reading frame, designated gltX, is very similar to bacterial glutamyl-tRNA synthetases and was demonstrated to be transcribed in vivo at 24 h postinfection by primer extension analysis. A lysine-rich open reading frame (LRO) of 117 codons was found upstream and divergent from gltX. The LRO lacks homology to known proteins, and we were unable to demonstrate that it is transcribed in vivo. The third open reading frame, of 182 codons, was found to be convergent with and partially overlap the LRO. It was confirmed to be preferentially expressed within the first 1.5 h of infection by Northern (RNA) blot analysis and was designated the early upstream open reading frame (EUO). Like the LRO, the EUO is not homologous to known proteins. A major potential transcription start site of the EUO was identified by primer extension analysis. However, the sequence upstream of the site does not closely resemble the consensus recognition sequences of bacterial sigma factors even though it is AT rich. The EUO is the first chlamydial gene specific to the early stage to be cloned and sequenced.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry C. E., 3rd, Hayes S. F., Hackstadt T. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science. 1992 Apr 17;256(5055):377–379. doi: 10.1126/science.256.5055.377. [DOI] [PubMed] [Google Scholar]
- Belunis C. J., Mdluli K. E., Raetz C. R., Nano F. E. A novel 3-deoxy-D-manno-octulosonic acid transferase from Chlamydia trachomatis required for expression of the genus-specific epitope. J Biol Chem. 1992 Sep 15;267(26):18702–18707. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Breton R., Sanfaçon H., Papayannopoulos I., Biemann K., Lapointe J. Glutamyl-tRNA synthetase of Escherichia coli. Isolation and primary structure of the gltX gene and homology with other aminoacyl-tRNA synthetases. J Biol Chem. 1986 Aug 15;261(23):10610–10617. [PubMed] [Google Scholar]
- Breton R., Watson D., Yaguchi M., Lapointe J. Glutamyl-tRNA synthetases of Bacillus subtilis 168T and of Bacillus stearothermophilus. Cloning and sequencing of the gltX genes and comparison with other aminoacyl-tRNA synthetases. J Biol Chem. 1990 Oct 25;265(30):18248–18255. [PubMed] [Google Scholar]
- Crenshaw R. W., Fahr M. J., Wichlan D. G., Hatch T. P. Developmental cycle-specific host-free RNA synthesis in Chlamydia spp. Infect Immun. 1990 Oct;58(10):3194–3201. doi: 10.1128/iai.58.10.3194-3201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Ganem D. A polymerase chain reaction-based approach to cloning sigma factors from eubacteria and its application to the isolation of a sigma-70 homolog from Chlamydia trachomatis. J Bacteriol. 1990 May;172(5):2447–2455. doi: 10.1128/jb.172.5.2447-2455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Ganem D. Chlamydial rRNA operons: gene organization and identification of putative tandem promoters. J Bacteriol. 1987 Dec;169(12):5678–5685. doi: 10.1128/jb.169.12.5678-5685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Pollack J., Malik F., Ganem D. Cloning and characterization of RNA polymerase core subunits of Chlamydia trachomatis by using the polymerase chain reaction. J Bacteriol. 1990 Oct;172(10):5732–5741. doi: 10.1128/jb.172.10.5732-5741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett K. D., Andersen A. A., Plaunt M., Hatch T. P. Cloning and sequence analysis of the major outer membrane protein gene of Chlamydia psittaci 6BC. Infect Immun. 1991 Aug;59(8):2853–2855. doi: 10.1128/iai.59.8.2853-2855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett K. D., Hatch T. P. Sequence analysis and lipid modification of the cysteine-rich envelope proteins of Chlamydia psittaci 6BC. J Bacteriol. 1991 Jun;173(12):3821–3830. doi: 10.1128/jb.173.12.3821-3830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahr M. J., Sriprakash K. S., Hatch T. P. Convergent and overlapping transcripts of the Chlamydia trachomatis 7.5-kb plasmid. Plasmid. 1992 Nov;28(3):247–257. doi: 10.1016/0147-619x(92)90056-g. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Al-Hossainy E., Silverman J. A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982 May;150(2):662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Miceli M., Sublett J. E. Synthesis of disulfide-bonded outer membrane proteins during the developmental cycle of Chlamydia psittaci and Chlamydia trachomatis. J Bacteriol. 1986 Feb;165(2):379–385. doi: 10.1128/jb.165.2.379-385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981 Apr;146(1):426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J. E., Burgess R. R., Thompson N. E., Stephens R. S. Chlamydia trachomatis RNA polymerase major sigma subunit. Sequence and structural comparison of conserved and unique regions with Escherichia coli sigma 70 and Bacillus subtilis sigma 43. J Biol Chem. 1990 Aug 5;265(22):13206–13214. [PubMed] [Google Scholar]
- Laberge S., Gagnon Y., Bordeleau L. M., Lapointe J. Cloning and sequencing of the gltX gene, encoding the glutamyl-tRNA synthetase of Rhizobium meliloti A2. J Bacteriol. 1989 Jul;171(7):3926–3932. doi: 10.1128/jb.171.7.3926-3932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Everson J. S., Ward M. E., Clarke I. N. Sulfur-rich proteins of Chlamydia trachomatis: developmentally regulated transcription of polycistronic mRNA from tandem promoters. Gene. 1990 Mar 1;87(1):105–112. doi: 10.1016/0378-1119(90)90500-q. [DOI] [PubMed] [Google Scholar]
- Mathews S. A., Douglas A., Sriprakash K. S., Hatch T. P. In vitro transcription in Chlamydia psittaci and Chlamydia trachomatis. Mol Microbiol. 1993 Mar;7(6):937–946. doi: 10.1111/j.1365-2958.1993.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., 5th Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987 Jan;55(1):162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara E., Ganem D., Engel J. N. A developmentally regulated chlamydial gene with apparent homology to eukaryotic histone H1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2125–2129. doi: 10.1073/pnas.89.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaunt M. R., Hatch T. P. Protein synthesis early in the developmental cycle of Chlamydia psittaci. Infect Immun. 1988 Dec;56(12):3021–3025. doi: 10.1128/iai.56.12.3021-3025.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988 Feb;170(2):744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Stephens R. S. Developmental-form-specific DNA-binding proteins in Chlamydia spp. Infect Immun. 1988 Jul;56(7):1678–1684. doi: 10.1128/iai.56.7.1678-1684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]






