Abstract
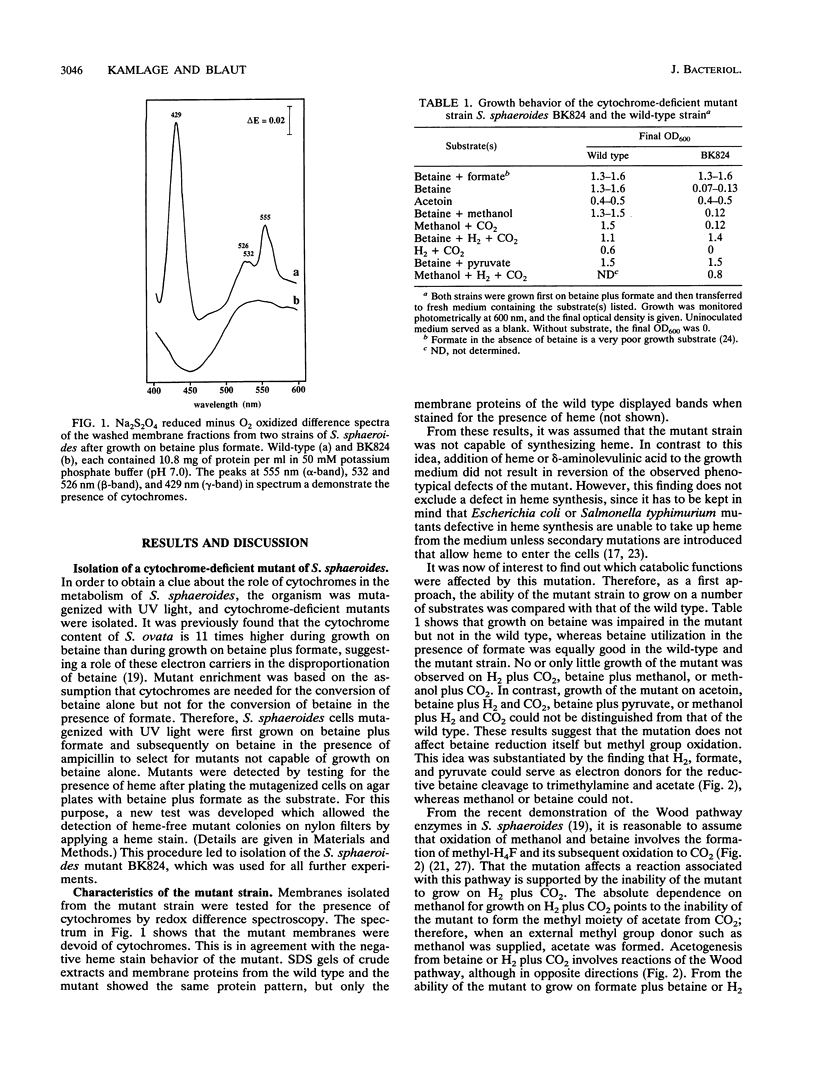
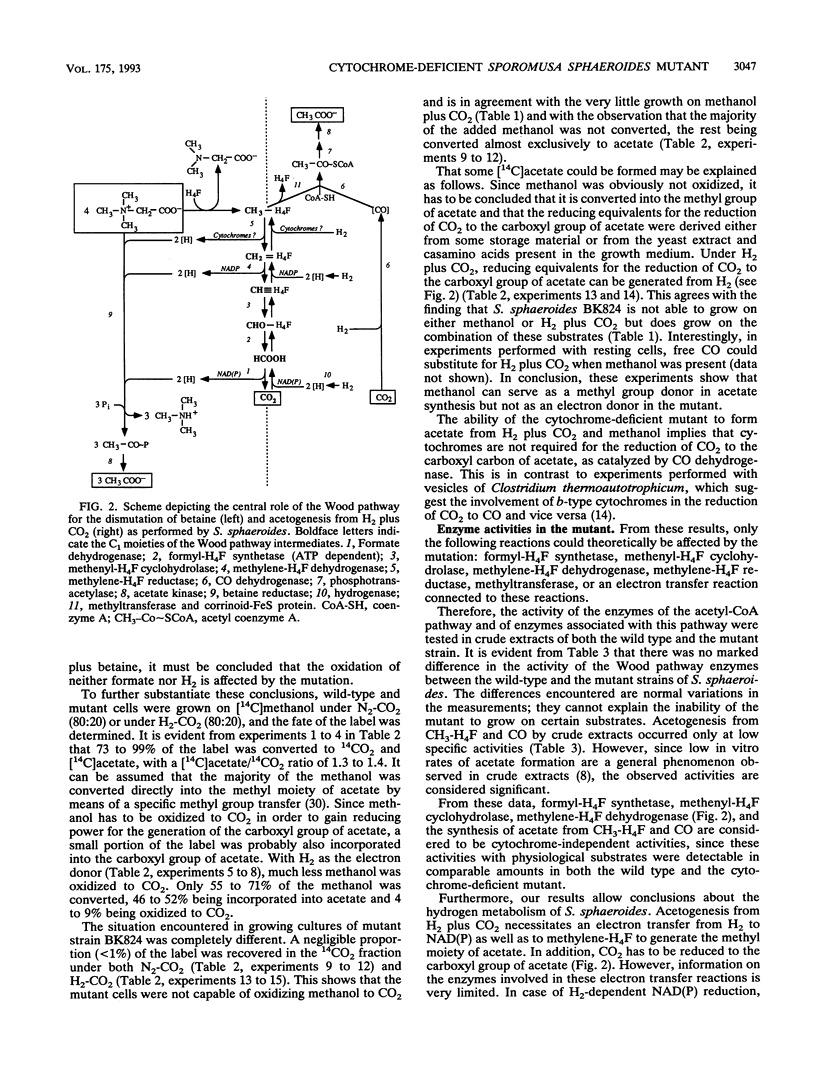
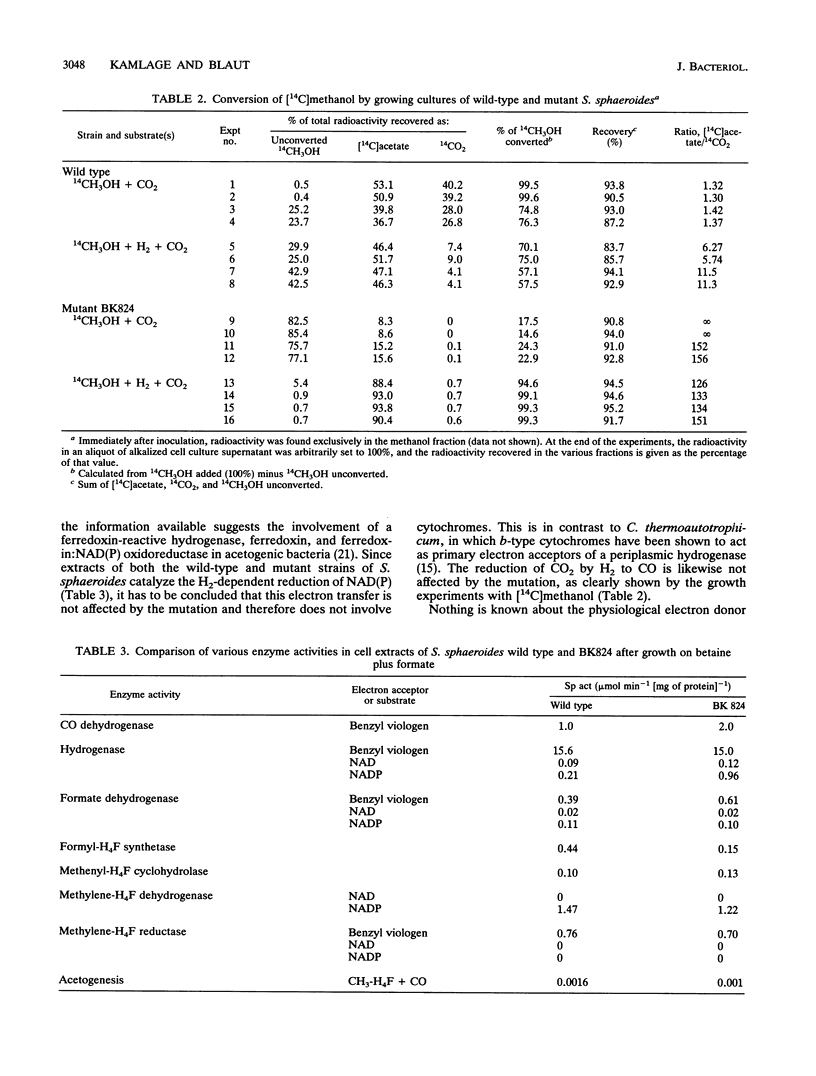
The homoacetogenic anaerobic bacterium Sporomusa sphaeroides was mutagenized with UV light. Taking advantage of the ampicillin enrichment technique and a newly developed test for the detection of heme in bacterial colonies, the cytochrome-deficient mutant strain S. sphaeroides BK824 was isolated. In contrast to the wild type, this mutant strain failed to grow on betaine, betaine plus methanol, H2 plus CO2, and methanol plus CO2. Growth on betaine plus formate, betaine plus H2, betaine plus pyruvate, methanol plus H2 and CO2, and acetoin was not impaired. All enzymes of the Wood pathway as well as hydrogenase and carbon monoxide dehydrogenase were detectable at comparable activities in both the wild type and the cytochrome-deficient mutant. Labeling experiments with [14C]methanol demonstrated the inability of S. sphaeroides BK824 to oxidize methyl groups. The role of cytochromes in electron transport steps associated with the Wood pathway enzymes and their possible role in energy conservation during autotrophic growth in acetogens are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B., Müller V., Gottschalk G. N5-methyl-tetrahydromethanopterin:coenzyme M methyltransferase of Methanosarcina strain Gö1 is an Na(+)-translocating membrane protein. J Bacteriol. 1992 Dec;174(23):7656–7660. doi: 10.1128/jb.174.23.7656-7660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichs D., Meyer M., Rieth M., Andreesen J. R. Interaction of selenoprotein PA and the thioredoxin system, components of the NADPH-dependent reduction of glycine in Eubacterium acidaminophilum and Clostridium litorale [corrected]. J Bacteriol. 1991 Oct;173(19):5983–5991. doi: 10.1128/jb.173.19.5983-5991.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn M., Andreesen J. R., Gottschalk G. Fermentation of fumarate and L-malate by Clostridium formicoaceticum. J Bacteriol. 1978 Jan;133(1):26–32. doi: 10.1128/jb.133.1.26-32.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Geerligs G., Schönheit P., Diekert G. Sodium dependent acetate formation from CO2 in Peptostreptococcus products (strain Marburg). FEMS Microbiol Lett. 1989 Feb;57(3):253–257. doi: 10.1016/0378-1097(89)90309-1. [DOI] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise R., Müller V., Gottschalk G. Presence of a sodium-translocating ATPase in membrane vesicles of the homoacetogenic bacterium Acetobacterium woodii. Eur J Biochem. 1992 Jun 1;206(2):553–557. doi: 10.1111/j.1432-1033.1992.tb16959.x. [DOI] [PubMed] [Google Scholar]
- Heise R., Müller V., Gottschalk G. Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium woodii. J Bacteriol. 1989 Oct;171(10):5473–5478. doi: 10.1128/jb.171.10.5473-5478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ivey D. M., Ljungdahl L. G. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol. 1987 Dec;169(12):5845–5847. doi: 10.1128/jb.169.12.5845-5847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ljungdahl L. G. Electron transport and electrochemical proton gradient in membrane vesicles of Clostridium thermoautotrophicum. J Bacteriol. 1989 May;171(5):2873–2875. doi: 10.1128/jb.171.5.2873-2875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ljungdahl L. G. Metabolism and energy generation in homoacetogenic clostridia. FEMS Microbiol Rev. 1990 Dec;7(3-4):383–389. doi: 10.1111/j.1574-6968.1990.tb04941.x. [DOI] [PubMed] [Google Scholar]
- Janzer J. J., Stan-Lotter H., Sanderson K. E. Isolation and characterization of hemin-permeable, envelope-defective mutants of Salmonella typhimurium. Can J Microbiol. 1981 Feb;27(2):226–237. doi: 10.1139/m81-034. [DOI] [PubMed] [Google Scholar]
- Kamlage B., Blaut M. Characterization of cytochromes from Methanosarcina strain Göl and their involvement in electron transport during growth on methanol. J Bacteriol. 1992 Jun;174(12):3921–3927. doi: 10.1128/jb.174.12.3921-3927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- McConville M. L., Charles H. P. Isolation of haemin-requiring mutants of Escherichia coli K12. J Gen Microbiol. 1979 Jul;113(1):155–164. doi: 10.1099/00221287-113-1-155. [DOI] [PubMed] [Google Scholar]
- Naumann E., Hippe H., Gottschalk G. Betaine: New Oxidant in the Stickland Reaction and Methanogenesis from Betaine and l-Alanine by a Clostridium sporogenes-Methanosarcina barkeri Coculture. Appl Environ Microbiol. 1983 Feb;45(2):474–483. doi: 10.1128/aem.45.2.474-483.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S. W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit Rev Biochem Mol Biol. 1991;26(3-4):261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Stupperich E., Aulkemeyer P., Eckerskorn C. Purification and characterization of a methanol-induced cobamide-containing protein from Sporomusa ovata. Arch Microbiol. 1992;158(5):370–373. doi: 10.1007/BF00245367. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wohlfarth G., Geerligs G., Diekert G. Purification and properties of a NADH-dependent 5,10-methylenetetrahydrofolate reductase from Peptostreptococcus productus. Eur J Biochem. 1990 Sep 11;192(2):411–417. doi: 10.1111/j.1432-1033.1990.tb19242.x. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Drake H. L. Differential effects of sodium on hydrogen- and glucose-dependent growth of the acetogenic bacterium Acetogenium kivui. Appl Environ Microbiol. 1990 Jan;56(1):81–86. doi: 10.1128/aem.56.1.81-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


