Abstract
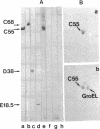
The DnaK protein of Zymomonas mobilis (DnaKz) was identified and found to be 80% identical to the DnaK protein of Escherichia coli on the basis of the sequence of the N-terminal 21 amino acids. The dnaKz gene was cloned and found to be expressed in a thermosensitive dnaK mutant of Escherichia coli. Expression of the foreign gene restored a thermoresistant phenotype but failed to modulate the heat shock response in E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan B., Linseman M., MacDonald L. A., Lam J. S., Kropinski A. M. Heat shock response of Pseudomonas aeruginosa. J Bacteriol. 1988 Aug;170(8):3668–3674. doi: 10.1128/jb.170.8.3668-3674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Functional domains of the Escherichia coli dnaK heat shock protein as revealed by mutational analysis. J Biol Chem. 1989 Dec 15;264(35):21122–21130. [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Delaney J. M. Requirement of the Escherichia coli dnaK gene for thermotolerance and protection against H2O2. J Gen Microbiol. 1990 Oct;136(10):2113–2118. doi: 10.1099/00221287-136-10-2113. [DOI] [PubMed] [Google Scholar]
- Diano M., Le Bivic A., Hirn M. A method for the production of highly specific polyclonal antibodies. Anal Biochem. 1987 Oct;166(1):224–229. doi: 10.1016/0003-2697(87)90568-9. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Hemmingsen S. M. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci. 1989 Aug;14(8):339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Tilly K., Drahos D., Hendrix R. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J Bacteriol. 1982 Mar;149(3):1175–1177. doi: 10.1128/jb.149.3.1175-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran P., Karunakaran T., Cami B., Mukundan A. G., Preziosi L., Baratti J. Cloning and sequencing of the sacA gene: characterization of a sucrase from Zymomonas mobilis. J Bacteriol. 1990 Dec;172(12):6727–6735. doi: 10.1128/jb.172.12.6727-6735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Fujita H., Wada M. High temperature induction of a stringent response in the dnaK(Ts) and dnaJ(Ts) mutants of Escherichia coli. J Biochem. 1986 Jun;99(6):1719–1724. doi: 10.1093/oxfordjournals.jbchem.a135648. [DOI] [PubMed] [Google Scholar]
- Karow M., Georgopoulos C. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J Bacteriol. 1992 Feb;174(3):702–710. doi: 10.1128/jb.174.3.702-710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K., Galitski T. P., Zylicz M., Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the sigma 32 transcription factor. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Michel G. P., Alvarez E., Guzzo J., Cami B., Baratti J. Cloning and expression in Escherichia coli of a phoA gene encoding a phosphate-irrepressible alkaline phosphatase of Zymomonas mobilis. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):103–108. doi: 10.1016/0378-1097(92)90139-f. [DOI] [PubMed] [Google Scholar]
- Michel G. P., Starka J. Effect of ethanol and heat stresses on the protein pattern of Zymomonas mobilis. J Bacteriol. 1986 Mar;165(3):1040–1042. doi: 10.1128/jb.165.3.1040-1042.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paek K. H., Walker G. C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987 Jan;169(1):283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]