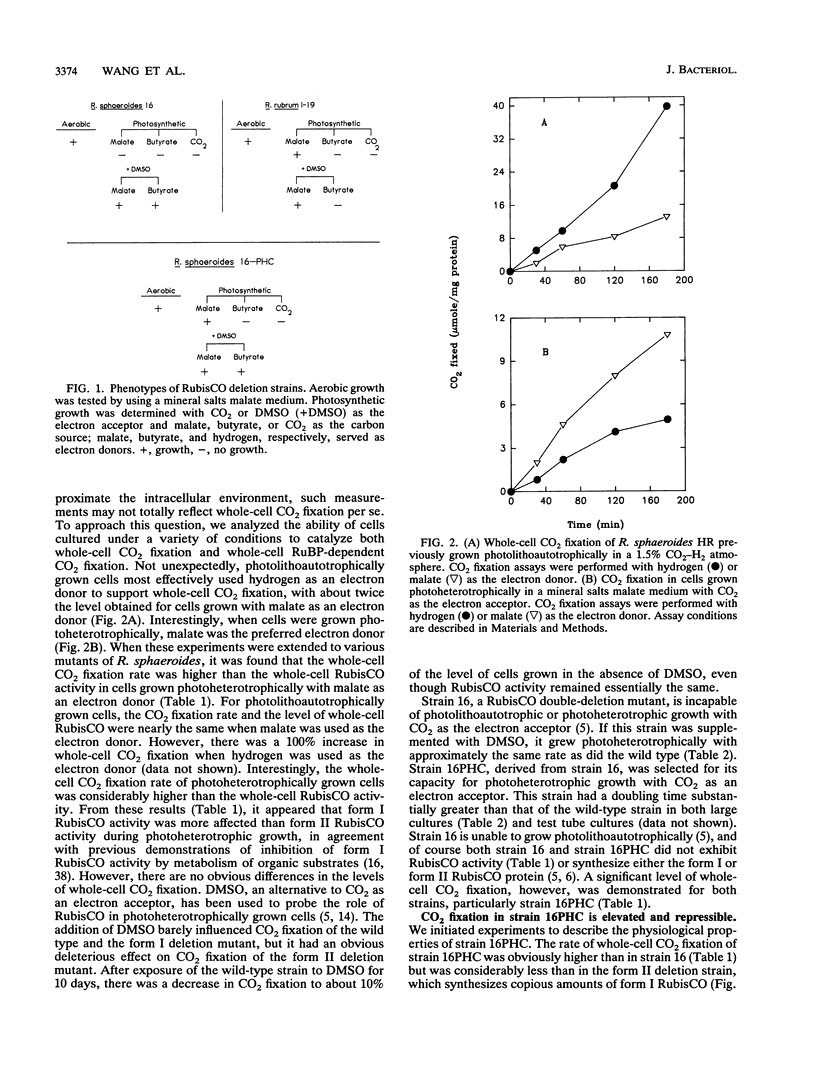
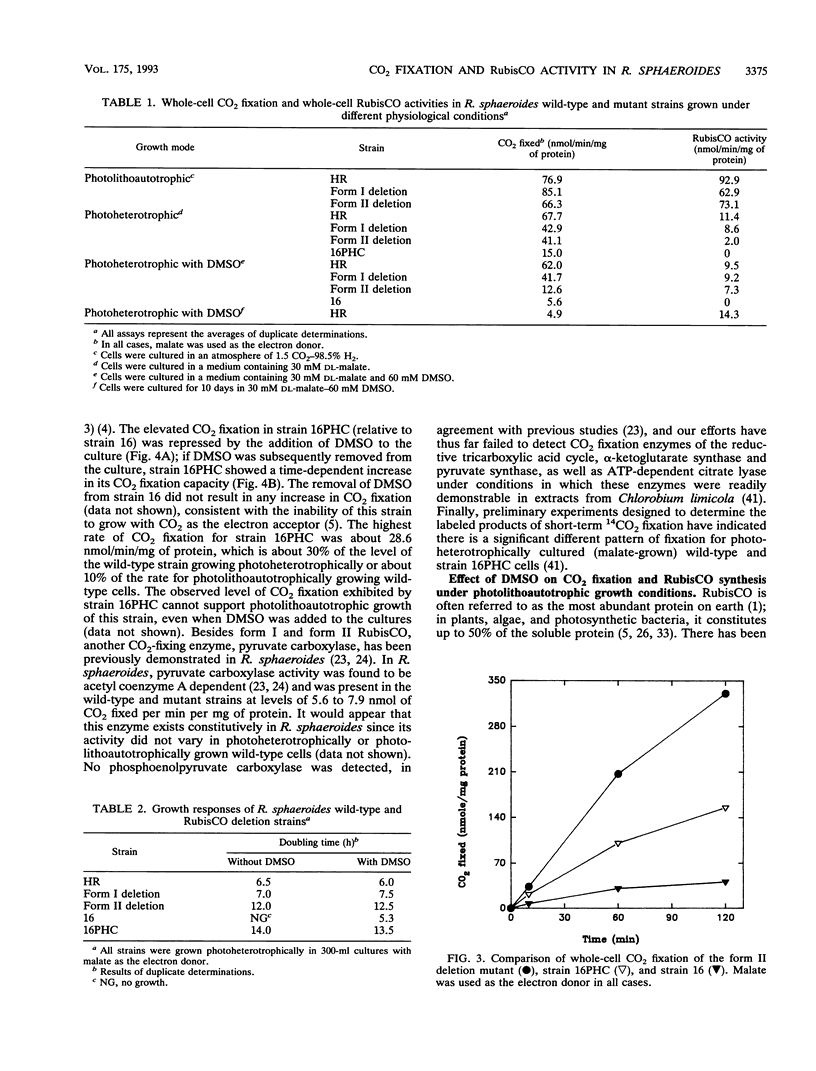
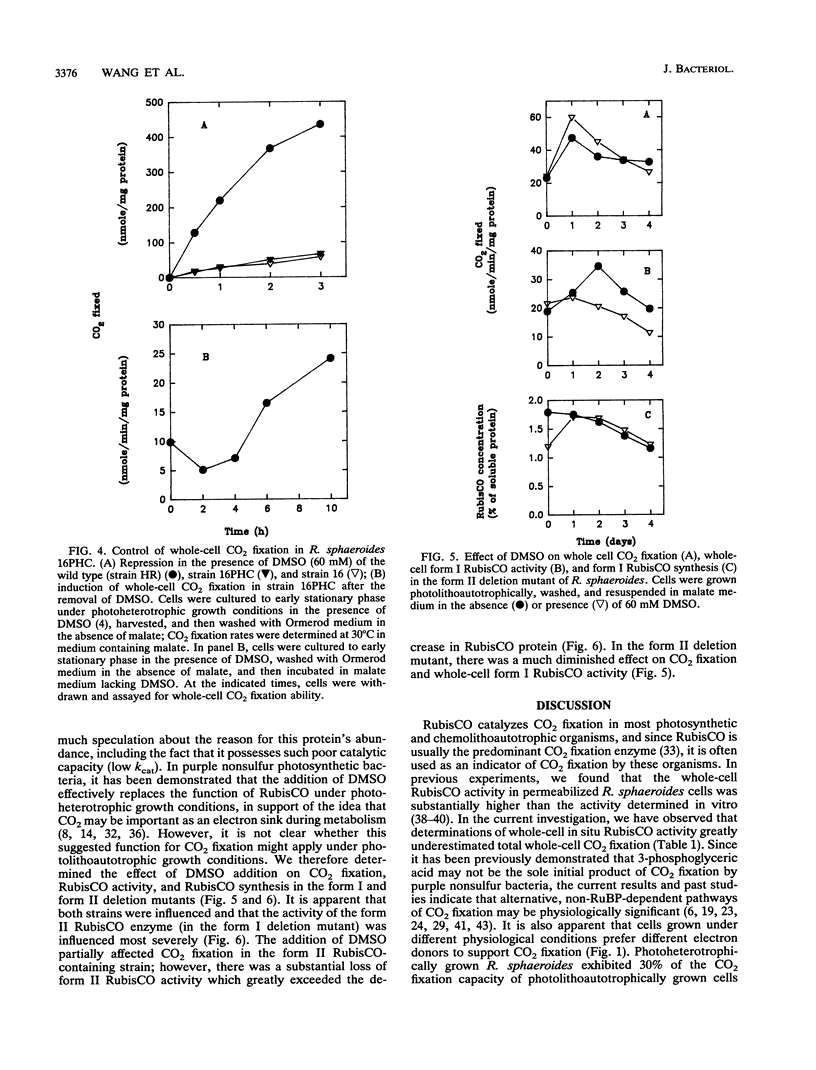
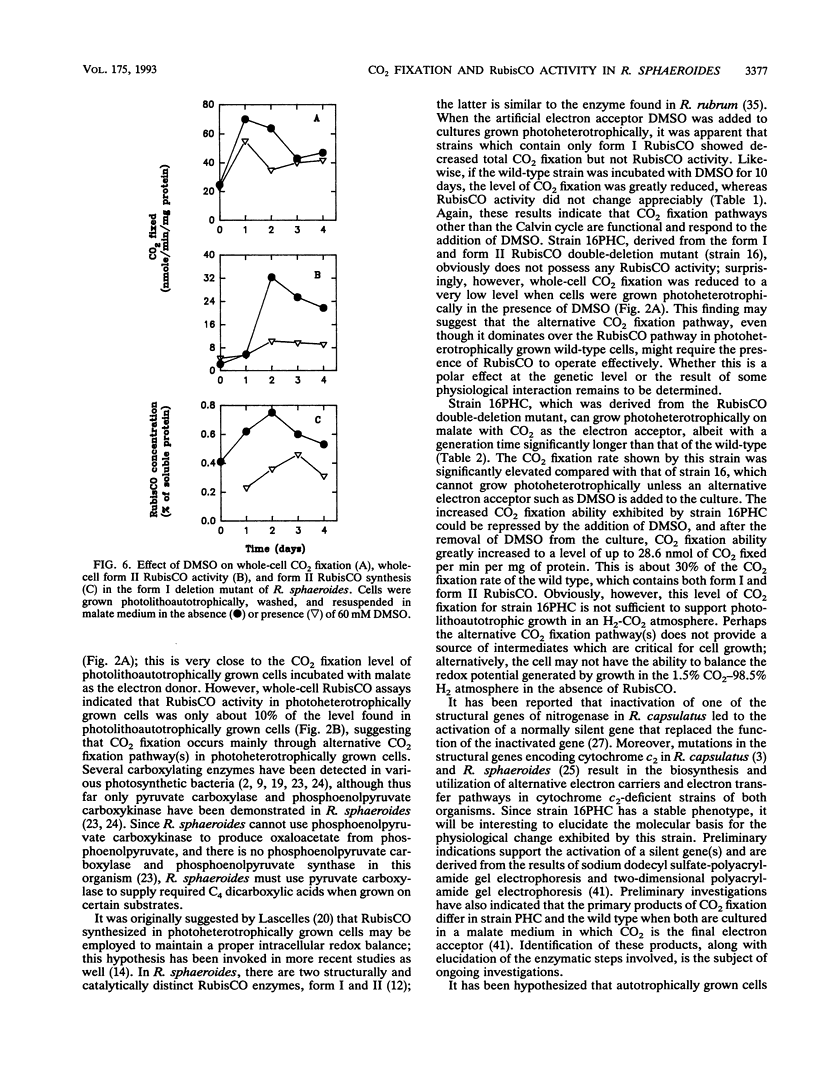
Abstract
Whole-cell CO2 fixation and ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) activity were determined in Rhodobacter sphaeroides wild-type and mutant strains. There is no obvious difference in the levels of whole-cell CO2 fixation for the wild type, a form I RubisCO deletion mutant, and a form II RubisCO deletion mutant. No ribulose 1,5-bisphosphate-dependent CO2 fixation was detected in a form I-form II RubisCO double-deletion mutant (strain 16) or strain 16PHC, a derivative from strain 16 which was selected for the ability to grow photoheterotrophically with CO2 as an electron acceptor. However, significant levels of whole-cell CO2 fixation were detected in both strains 16 and 16PHC. Strain 16PHC exhibited CO2 fixation rates significantly higher than those of strain 16; the rates found for strain 16PHC were 30% of the level found in photoheterotrophically grown wild-type strain HR containing both form I and form II RubisCO and 10% of the level of the wild-type strain grown photolithoautotrophically. Strain 16PHC could not grow photolithoautotrophically in a CO2-H2 atmosphere; however, CO2 fixation catalyzed by photoheterotrophically grown strain 16PHC was repressed by addition of the alternate electron acceptor dimethyl sulfoxide. Dimethyl sulfoxide addition also influenced RubisCO activity under photolithoautotrophic conditions; 40 to 70% of the RubisCO activity was reduced without significantly influencing growth. Strain 16PHC and strain 16 contain nearly equivalent but low levels of pyruvate carboxylase, indicating that CO2 fixation enzymes other than pyruvate carboxylase contribute to the ability of strain 16PHC to grow with CO2 as an electron acceptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan B. B., Evans M. C., Arnon D. I. Ferredoxin-dependent carbon assimilation in Rhodospirillum rubrum. Arch Mikrobiol. 1967;59(1):32–40. doi: 10.1007/BF00406314. [DOI] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. L., Quivey R. G., Jr, Tabita F. R. Transposon mutagenesis and physiological analysis of strains containing inactivated form I and form II ribulose bisphosphate carboxylase/oxygenase genes in Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):5–11. doi: 10.1128/jb.170.1.5-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. L., Tabita F. R. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J Bacteriol. 1991 Mar;173(6):2099–2108. doi: 10.1128/jb.173.6.2099-2108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Falcone D. L., Tabita F. R. Nucleotide sequence, transcriptional analysis, and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Aug 5;266(22):14646–14653. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Structural differences in the catalytic subunits of form I and form II ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Dec;164(3):1188–1193. doi: 10.1128/jb.164.3.1188-1193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Roles of CfxA, CfxB, and external electron acceptors in regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase expression in Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1736–1748. doi: 10.1128/jb.172.4.1736-1748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. In vivo regulation of form I ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1987 Apr;254(1):290–303. doi: 10.1016/0003-9861(87)90105-6. [DOI] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT M. The photometabolism of propionate by Rhodospirillum rubrum. Biochem J. 1962 Jul;84:170–185. doi: 10.1042/bj0840170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The formation of ribulose 1:5-diphosphate carboxylase by growing cultures of Athiorhodaceae. J Gen Microbiol. 1960 Dec;23:499–510. doi: 10.1099/00221287-23-3-499. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madigan M. T., Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol. 1979 Jan;137(1):524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J., Morris J. G. Acetate utilisation by Rhodopseudomonas spheroides. FEBS Lett. 1969 Jul;4(1):52–54. doi: 10.1016/0014-5793(69)80194-8. [DOI] [PubMed] [Google Scholar]
- Payne J., Morris J. G. Pyruvate carboxylase in Rhodopseudomonas spheroides. J Gen Microbiol. 1969 Nov;59(1):97–101. doi: 10.1099/00221287-59-1-97. [DOI] [PubMed] [Google Scholar]
- Rott M. A., Donohue T. J. Rhodobacter sphaeroides spd mutations allow cytochrome c2-independent photosynthetic growth. J Bacteriol. 1990 Apr;172(4):1954–1961. doi: 10.1128/jb.172.4.1954-1961.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarles L. S., Tabita F. R. Derepression of the synthesis of D-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J Bacteriol. 1983 Jan;153(1):458–464. doi: 10.1128/jb.153.1.458-464.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R., Caruso P., Whitman W. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal Biochem. 1978 Feb;84(2):462–472. doi: 10.1016/0003-2697(78)90064-7. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J Biol Chem. 1974 Jun 10;249(11):3453–3458. [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R. Pyridine nucleotide control and subunit structure of phosphoribulokinase from photosynthetic bacteria. J Bacteriol. 1980 Sep;143(3):1275–1280. doi: 10.1128/jb.143.3.1275-1280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tabita F. R. Interaction between ribulose 1,5-bisphosphate carboxylase/oxygenase activity and the ammonia assimilatory system of Rhodobacter sphaeroides. J Bacteriol. 1992 Jun;174(11):3601–3606. doi: 10.1128/jb.174.11.3601-3606.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tabita F. R. Interaction of inactivated and active ribulose 1,5-bisphosphate carboxylase/oxygenase of Rhodobacter sphaeroides with nucleotides and the chaperonin 60 (GroEL) protein. J Bacteriol. 1992 Jun;174(11):3607–3611. doi: 10.1128/jb.174.11.3607-3611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tabita F. R. Reversible inactivation and characterization of purified inactivated form I ribulose 1,5-bisphosphate carboxylase/oxygenase of Rhodobacter sphaeroides. J Bacteriol. 1992 Jun;174(11):3593–3600. doi: 10.1128/jb.174.11.3593-3600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Tabita F. R. Isolation and partial characterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983 Nov;156(2):507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Growth of Rhodopseudomonas capsulata under anaerobic dark conditions with dimethyl sulfoxide. Arch Biochem Biophys. 1977 Jun;181(2):411–418. doi: 10.1016/0003-9861(77)90246-6. [DOI] [PubMed] [Google Scholar]


