Abstract
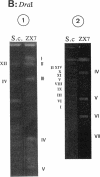
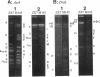
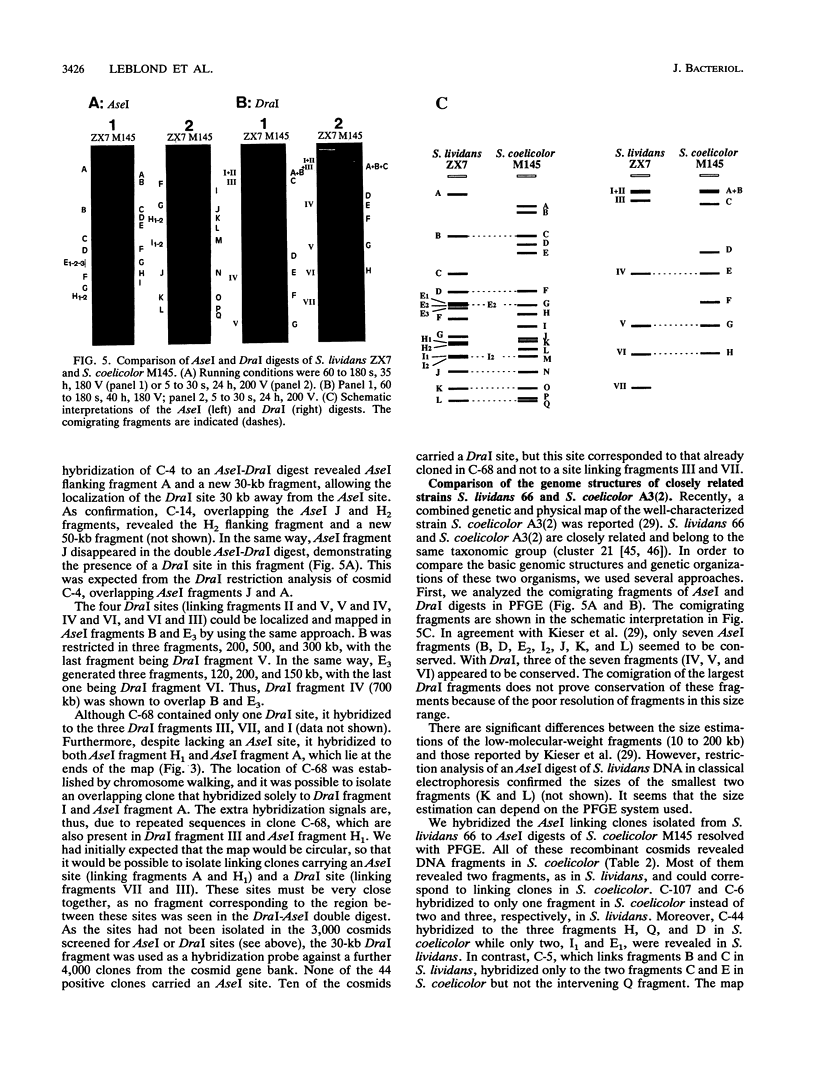
A physical map of the chromosome of Streptomyces lividans 66 ZX7 was constructed by ordering the macrorestriction fragments generated from the genomic DNA with the restriction enzymes AseI and DraI. AseI and DraI linking cosmids (i.e., recombinant cosmids including either AseI or DraI sites) were isolated from a gene bank and used as hybridization probes against Southern transfers of pulsed-field gel electrophoresis (PFGE) restriction patterns. The DraI sites were precisely mapped by PFGE analyses of AseI-DraI double digests and hybridization with the AseI junctions. The 16 AseI and 7 DraI fragments were aligned as a single chromosome of about 8,000 kb. The data supported the interpretation that the chromosome is a linear structure. The related strain Streptomyces coelicolor A3(2) M145, recently mapped by H. Kieser, T. Kieser, and D. A. Hopwood (J. Bacteriol. 174:5496-5507, 1992), was compared with S. lividans at the level of the genomic structure by hybridizing the linking cosmids to Southern transfers of PFGE patterns. In spite of little apparent similarity in their restriction patterns, the comparison of the physical maps revealed a common structure with an identical ordering of the cosmid sequences. This conservation of the map order was further confirmed by assigning genetic markers (i.e., cloned genes and DNA elements relevant to the unstable region) to the AseI fragments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Cullum J. DNA amplification and an unstable arginine gene in Streptomyces lividans 66. Mol Gen Genet. 1984;195(1-2):134–138. doi: 10.1007/BF00332735. [DOI] [PubMed] [Google Scholar]
- Baylis H. A., Bibb M. J. Organisation of the ribosomal RNA genes in Streptomyces coelicolor A3(2). Mol Gen Genet. 1988 Feb;211(2):191–196. doi: 10.1007/BF00330593. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor C. R., Smith C. L., Mathew M. K. Pulsed-field gel electrophoresis of very large DNA molecules. Annu Rev Biophys Biophys Chem. 1988;17:287–304. doi: 10.1146/annurev.bb.17.060188.001443. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Davis N. K., Chater K. F. Spore colour in Streptomyces coelicolor A3(2) involves the developmentally regulated synthesis of a compound biosynthetically related to polyketide antibiotics. Mol Microbiol. 1990 Oct;4(10):1679–1691. doi: 10.1111/j.1365-2958.1990.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Davis N. K., Chater K. F. The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Mol Gen Genet. 1992 Apr;232(3):351–358. doi: 10.1007/BF00266237. [DOI] [PubMed] [Google Scholar]
- Dyson P., Schrempf H. Genetic instability and DNA amplification in Streptomyces lividans 66. J Bacteriol. 1987 Oct;169(10):4796–4803. doi: 10.1128/jb.169.10.4796-4803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Lewis K., Rothenberg B. E. High efficiency vectors for cosmid microcloning and genomic analysis. Gene. 1989 Jun 30;79(1):9–20. doi: 10.1016/0378-1119(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Feitelson J. S., Malpartida F., Hopwood D. A. Genetic and biochemical characterization of the red gene cluster of Streptomyces coelicolor A3(2). J Gen Microbiol. 1985 Sep;131(9):2431–2441. doi: 10.1099/00221287-131-9-2431. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Harasym M., Zhang L. H., Chater K., Piret J. The Streptomyces coelicolor A3(2) bldB region contains at least two genes involved in morphological development. J Gen Microbiol. 1990 Aug;136(8):1543–1550. doi: 10.1099/00221287-136-8-1543. [DOI] [PubMed] [Google Scholar]
- Henderson D. J., Lydiate D. J., Hopwood D. A. Structural and functional analysis of the mini-circle, a transposable element of Streptomyces coelicolor A3(2). Mol Microbiol. 1989 Oct;3(10):1307–1318. doi: 10.1111/j.1365-2958.1989.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Hercomb J., Thierbach G., Baumberg S., Parish J. H. Cloning, characterization and expression in Escherichia coli of a leucine biosynthetic gene from Streptomyces rochei. J Gen Microbiol. 1987 Feb;133(2):317–322. doi: 10.1099/00221287-133-2-317. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Häusler A., Birch A., Krek W., Piret J., Hütter R. Heterogeneous genomic amplification in Streptomyces glaucescens: structure, location and junction sequence analysis. Mol Gen Genet. 1989 Jun;217(2-3):437–446. doi: 10.1007/BF02464915. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Nakano M. M., Ogawara H. Cloning of a gene from Streptomyces species complementing argG mutations. J Antibiot (Tokyo) 1985 Jun;38(6):787–794. doi: 10.7164/antibiotics.38.787. [DOI] [PubMed] [Google Scholar]
- Kendall K., Ali-Dunkrah U., Cullum J. Cloning of the galactokinase gene (galK) from Streptomyces coelicolor A3(2). J Gen Microbiol. 1987 Mar;133(3):721–725. doi: 10.1099/00221287-133-3-721. [DOI] [PubMed] [Google Scholar]
- Kieser H. M., Kieser T., Hopwood D. A. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J Bacteriol. 1992 Sep;174(17):5496–5507. doi: 10.1128/jb.174.17.5496-5507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi H., Shimaji M., Sakai A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. 1987 Jul 30-Aug 5Nature. 328(6129):454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- Leblond P., Demuyter P., Simonet J. M., Decaris B. Genetic instability and associated genome plasticity in Streptomyces ambofaciens: pulsed-field gel electrophoresis evidence for large DNA alterations in a limited genomic region. J Bacteriol. 1991 Jul;173(13):4229–4233. doi: 10.1128/jb.173.13.4229-4233.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond P., Demuyter P., Simonet J. M., Decaris B. Genetic instability and hypervariability in Streptomyces ambofaciens: towards an understanding of a mechanism of genome plasticity. Mol Microbiol. 1990 May;4(5):707–714. doi: 10.1111/j.1365-2958.1990.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Leblond P., Francou F. X., Simonet J. M., Decaris B. Pulsed-field gel electrophoresis analysis of the genome of Streptomyces ambofaciens strains. FEMS Microbiol Lett. 1990 Oct;60(1-2):79–88. doi: 10.1016/0378-1097(90)90349-u. [DOI] [PubMed] [Google Scholar]
- Mendez C., Chater K. F. Cloning of whiG, a gene critical for sporulation of Streptomyces coelicolor A3(2). J Bacteriol. 1987 Dec;169(12):5715–5720. doi: 10.1128/jb.169.12.5715-5720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret J. M., Chater K. F. Phage-mediated cloning of bldA, a region involved in Streptomyces coelicolor morphological development, and its analysis by genetic complementation. J Bacteriol. 1985 Sep;163(3):965–972. doi: 10.1128/jb.163.3.965-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plohl M., Gamulin V. Sequence of the 5S rRNA gene and organization of ribosomal RNA operons in Streptomyces rimosus. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):139–143. doi: 10.1016/0378-1097(91)90541-h. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Seno E. T., Bruton C. J., Chater K. F. The glycerol utilization operon of Streptomyces coelicolor: genetic mapping of gyl mutations and the analysis of cloned gylDNA. Mol Gen Genet. 1984;193(1):119–128. doi: 10.1007/BF00327424. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Ono Y., Nagata A., Yamada T. Molecular cloning and characterization of an rRNA operon in Streptomyces lividans TK21. J Bacteriol. 1988 Apr;170(4):1631–1636. doi: 10.1128/jb.170.4.1631-1636.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. T., Goodfellow M., Alderson G., Wellington E. M., Sneath P. H., Sackin M. J. Numerical classification of Streptomyces and related genera. J Gen Microbiol. 1983 Jun;129(6):1743–1813. doi: 10.1099/00221287-129-6-1743. [DOI] [PubMed] [Google Scholar]
- Zhou X., Deng Z., Firmin J. L., Hopwood D. A., Kieser T. Site-specific degradation of Streptomyces lividans DNA during electrophoresis in buffers contaminated with ferrous iron. Nucleic Acids Res. 1988 May 25;16(10):4341–4352. doi: 10.1093/nar/16.10.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]