Abstract
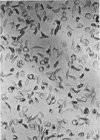
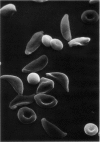
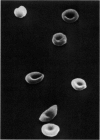
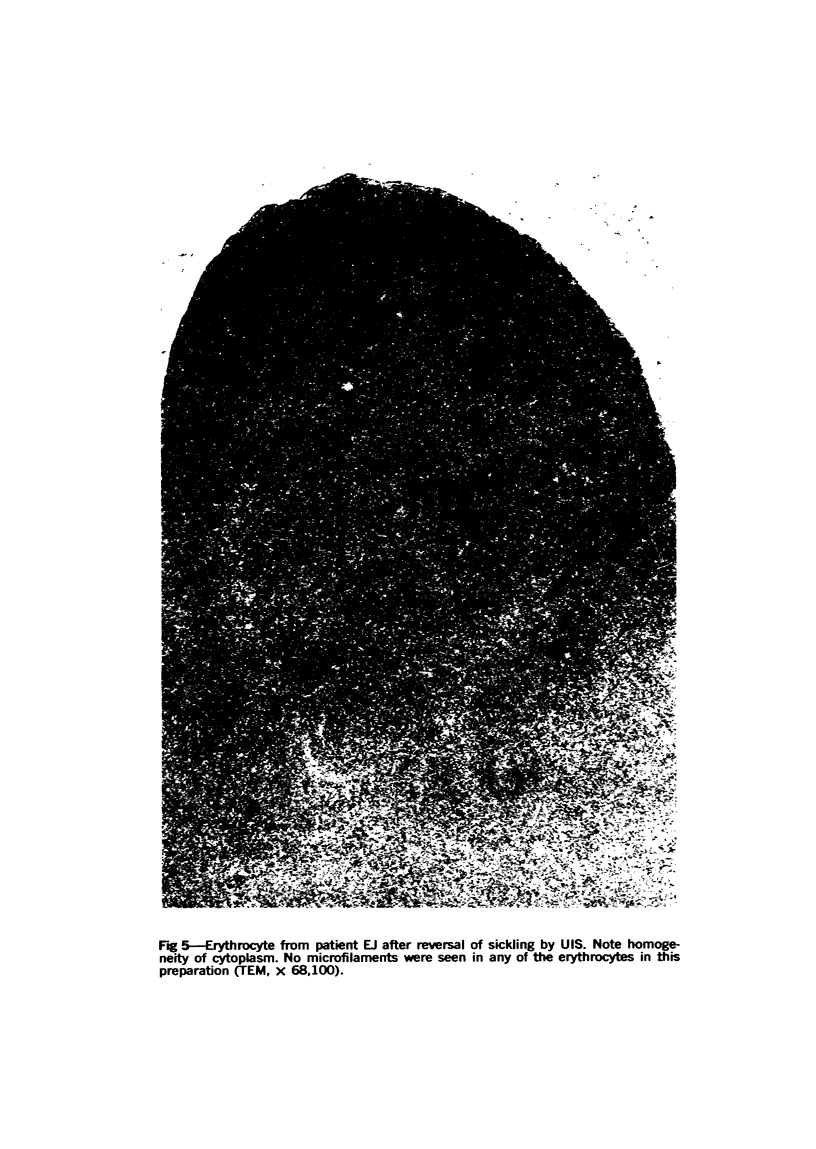
Optical and electron microscopic evidence is presented to support the finding that sickling of hemoglobin S can be reversed and blocked by urea in invert sugar (UIS). Erythrocytes from subjects having hemoglobin SS, AS or AA were treated with the UIS either before or after deoxygenation with Na2S2O5. Light microscopic studies indicated that approximately one-fifth as much urea is required to block sickling as is necessary to reverse sickled poikilocytes to normal forms. Intracellular microfilaments apparent in transmission electron micrographs of sickled erythrocytes were eliminated by treating aliquots of the same deoxygenated erythrocytes with UIS. Scanning electron micrographs showed a reversion of sickled poikilocytes to a normal erythrocyte population of biconcave discs. The use of UIS was deduced from Murayama's hypothesis that the molecular mechanism of sickling clearly involves hydrophobic bonds formed between the number-6 valine substitution of the β-chain S globins and the α-chain globins of interacting hemoglobin molecules. The use of UIS to arrest the formation of such hydrophobic bonds is advocated as an evident and effective therapeutic strategy to treat sickle cell crisis.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertles J. F., Döbler J. Reversible and irreversible sickling: a distinction by electron microscopy. Blood. 1969 Jun;33(6):884–898. [PubMed] [Google Scholar]
- Clarke J. A., Salsbury A. J. Surface ultramicroscopy of human blood cells. Nature. 1967 Jul 22;215(5099):402–404. doi: 10.1038/215402a0. [DOI] [PubMed] [Google Scholar]
- Farnsworth P. N., Nadel M. R., Stoll B. J. Surface ultramicroscopy of sickle cells. Nature. 1970 Jan 10;225(5228):190–191. doi: 10.1038/225190a0. [DOI] [PubMed] [Google Scholar]
- GILBOE D., JAVID M. Electrolyte studies following a single administration of hypertonic urea. Surg Gynecol Obstet. 1963 Jun;116:693–700. [PubMed] [Google Scholar]
- JAVID M. Urea; new use of an old agent; reduction of intracranial and intraocular pressure. Surg Clin North Am. 1958 Aug;38(4):907–928. doi: 10.1016/s0039-6109(16)35526-8. [DOI] [PubMed] [Google Scholar]
- Jensen W. N. Fragmentation and the "freakish poikilocyte". Am J Med Sci. 1969 Jun;257(6):355–364. doi: 10.1097/00000441-196906000-00001. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Murayama M. Structure of sickle cell hemoglobin and molecular mechanism of the sickling phenomenon. Clin Chem. 1967 Jul;13(7):578–588. [PubMed] [Google Scholar]
- Nalbandian R. M., Henry R. L., Nichols B. M., Camp F. R., Jr, Wolf P. L. Molecular basis for a simple, specific test for S hemoglobin: the Murayama test. Clin Chem. 1970 Nov;16(11):945–950. [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONDER E., PONDER R. Fragmentation of red cells and ghosts in urea solutions, with a note on electrophoresis patterns. Acta Haematol. 1954 Oct;12(4):282–290. doi: 10.1159/000204630. [DOI] [PubMed] [Google Scholar]
- Párducz B. Ciliary movement and coordination in ciliates. Int Rev Cytol. 1967;21:91–128. doi: 10.1016/s0074-7696(08)60812-8. [DOI] [PubMed] [Google Scholar]
- REBUCK J. W., STURROCK R. M., MONTO R. W. Sequential electron micrography of sickling. Lab Invest. 1955 May-Jun;4(3):175–189. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., MILLER F., BARRNETT R. J. ALDEHYDE FIXATION FOR MORPHOLOGICAL AND ENZYME HISTOCHEMICAL STUDIES WITH THE ELECTRON MICROSCOPE. J Histochem Cytochem. 1964 Feb;12:57–71. doi: 10.1177/12.2.57. [DOI] [PubMed] [Google Scholar]
- Stetson C. A., Jr The state of hemoglobin in sickled erythrocytes. J Exp Med. 1966 Feb 1;123(2):341–346. doi: 10.1084/jem.123.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEG D. E., BECKER C., BEARD J. W. VIRUS OF AVIAN MYELOBLASTOSIS (BAI STRAIN A). XXV. ULTRACYTOCHEMICAL STUDY OF VIRUS AND MYELOBLAST PHOSPHATASE ACTIVITY. J Natl Cancer Inst. 1964 Jan;32:201–235. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. Solubility of amino acids in aqueous urea solutions and its implications for the denaturation of proteins by urea. J Biol Chem. 1962 May;237:1735–1737. [PubMed] [Google Scholar]
- White J. G. The fine structure of sickled hemoglobin in situ. Blood. 1968 May;31(5):561–579. [PubMed] [Google Scholar]