Abstract
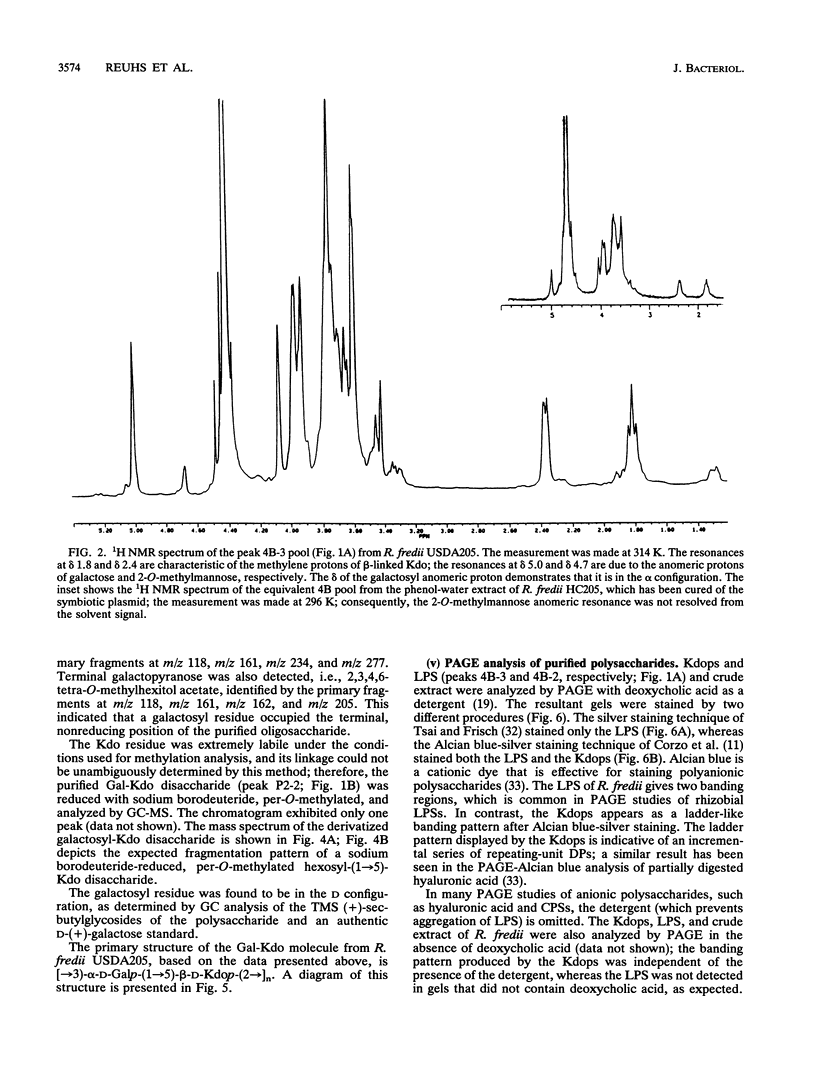
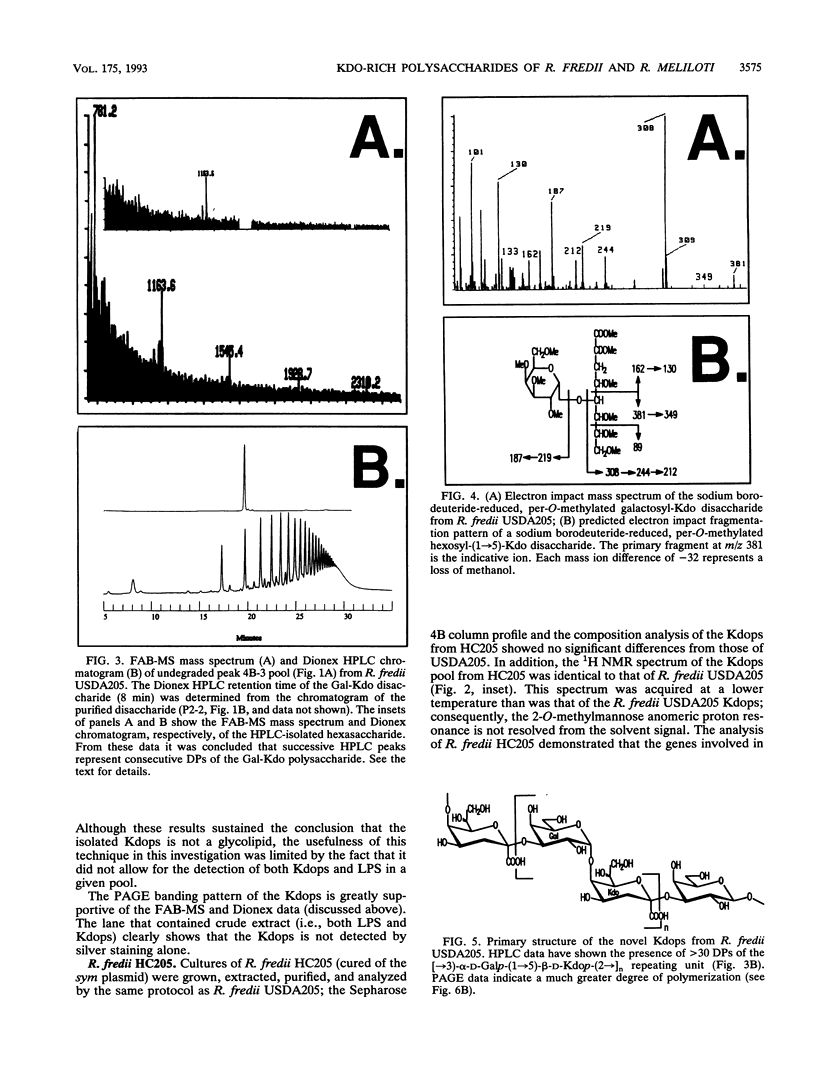
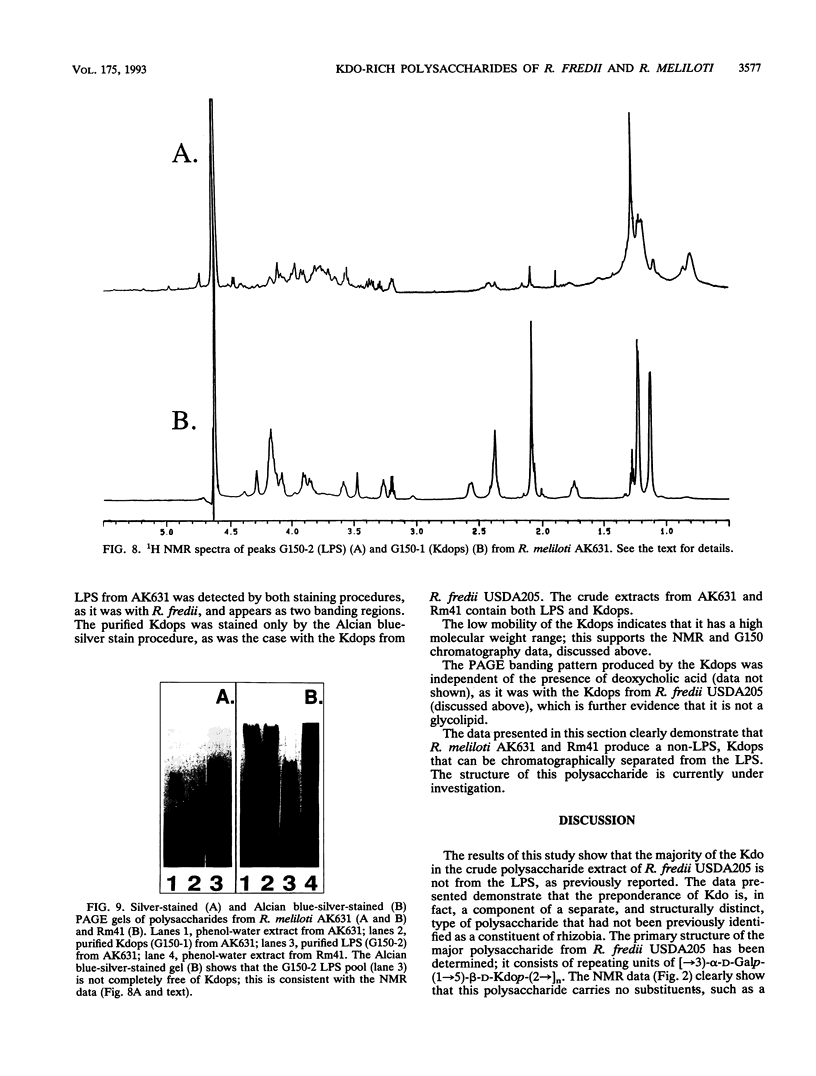
The polysaccharide components from cultured cells of Rhizobium fredii USDA205 and Rhizobium meliloti AK631 were extracted with hot phenol-water and separated by repetitive gel filtration chromatography. Polyacrylamide gel electrophoresis, nuclear magnetic resonance spectrometry, and gas chromatography analyses showed that both of these bacterial species produce unique polysaccharides that contain a high proportion of 3-deoxy-D-manno-2-octulosonic acid (Kdo). These polysaccharides, which constituted a major portion of the extracted carbohydrate, are not excreted into the growth media (i.e., they are not extracellular polysaccharides) and are structurally distinct from the lipopolysaccharides. The primary structure of the preponderant polysaccharide from R. fredii USDA205 was determined by high-performance anion-exchange liquid chromatography, nuclear magnetic resonance spectrometry, fast atom bombardment-mass spectrometry, and gas chromatography-mass spectrometry; it consists of repeating units of [-->3)-alpha-D-Galp-(1-->5)-beta-D-Kdop-(2-->]n. This molecule is structurally analogous to the constituents of one subgroup of K antigens (capsular polysaccharides) produced by Escherichia coli. Polysaccharides of this type have not previously been identified as components of rhizobial cells. The Kdo-containing polysaccharide from R. meliloti, which has not been completely characterized, appears to be structurally related to that of R. fredii.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cangelosi G. A., Hung L., Puvanesarajah V., Stacey G., Ozga D. A., Leigh J. A., Nester E. W. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J Bacteriol. 1987 May;169(5):2086–2091. doi: 10.1128/jb.169.5.2086-2091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Kalembasa S., Turowski D., Pachori P., Noel K. D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987 Nov;169(11):4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Yadav M. Isolation and partial characterization of the extracellular polysaccharides and lipopolysaccharides from fast-growing Rhizobium japonicum USDA 205 and its Nod- mutant, HC205, which lacks the symbiotic plasmid. Appl Environ Microbiol. 1985 Nov;50(5):1219–1224. doi: 10.1128/aem.50.5.1219-1224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion M., Bhat U. R., Reuhs B., Carlson R. W. Isolation and characterization of the lipopolysaccharides from Bradyrhizobium japonicum. J Bacteriol. 1990 Apr;172(4):1725–1731. doi: 10.1128/jb.172.4.1725-1731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo J., Pérez-Galdona R., León-Barrios M., Gutiérrez-Navarro A. M. Alcian blue fixation allows silver staining of the isolated polysaccharide component of bacterial lipopolysaccharides in polyacrylamide gels. Electrophoresis. 1991 Jun;12(6):439–441. doi: 10.1002/elps.1150120611. [DOI] [PubMed] [Google Scholar]
- Diebold R., Noel K. D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989 Sep;171(9):4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotter G. S., Scott D. B. Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an indeterminate nodulating host. J Bacteriol. 1991 Jan;173(2):851–859. doi: 10.1128/jb.173.2.851-859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Jann K., Jann B. Biochemistry and expression of bacterial capsules. Biochem Soc Trans. 1991 Aug;19(3):623–628. doi: 10.1042/bst0190623. [DOI] [PubMed] [Google Scholar]
- Jarvis B. D., Downer H. L., Young J. P. Phylogeny of fast-growing soybean-nodulating rhizobia support synonymy of Sinorhizobium and Rhizobium and assignment to Rhizobium fredii. Int J Syst Bacteriol. 1992 Jan;42(1):93–96. doi: 10.1099/00207713-42-1-93. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Tully R. E., Keister D. L. Exopolysaccharide-Deficient Mutants of Rhizobium fredii HH303 Which Are Symbiotically Effective. Appl Environ Microbiol. 1989 Jul;55(7):1852–1854. doi: 10.1128/aem.55.7.1852-1854.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenter M., Jann B., Jann K. Structure of the K16 antigen from Escherichia coli O7:K16:H-, a Kdo-containing capsular polysaccharide. Carbohydr Res. 1990 Mar 25;197:197–204. doi: 10.1016/0008-6215(90)84142-h. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnoky P., Grosskopf E., Ha D. T., Kiss G. B., Kondorosi A. Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J Cell Biol. 1988 Mar;106(3):597–607. doi: 10.1083/jcb.106.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnoky P., Kondorosi A. Two gene clusters of Rhizobium meliloti code for early essential nodulation functions and a third influences nodulation efficiency. J Bacteriol. 1986 Sep;167(3):881–887. doi: 10.1128/jb.167.3.881-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnoky P., Petrovics G., Kereszt A., Grosskopf E., Ha D. T., Bánfalvi Z., Kondorosi A. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol. 1990 Sep;172(9):5450–5458. doi: 10.1128/jb.172.9.5450-5458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber T. L., Reed J., Glazebrook J., Glucksmann M. A., Ahmann D., Marra A., Walker G. C. Rhizobium meliloti exopolysaccharides: genetic analyses and symbiotic importance. Biochem Soc Trans. 1991 Aug;19(3):636–641. doi: 10.1042/bst0190636. [DOI] [PubMed] [Google Scholar]
- Stacey G., So J. S., Roth L. E., Lakshmi SK B., Carlson R. W. A lipopolysaccharide mutant of Bradyrhizobium japonicum that uncouples plant from bacterial differentiation. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):332–340. doi: 10.1094/mpmi-4-332. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Turner R. E., Cowman M. K. Cationic dye binding by hyaluronate fragments: dependence on hyaluronate chain length. Arch Biochem Biophys. 1985 Feb 15;237(1):253–260. doi: 10.1016/0003-9861(85)90276-0. [DOI] [PubMed] [Google Scholar]
- Williams M. N., Hollingsworth R. I., Brzoska P. M., Signer E. R. Rhizobium meliloti chromosomal loci required for suppression of exopolysaccharide mutations by lipopolysaccharide. J Bacteriol. 1990 Nov;172(11):6596–6598. doi: 10.1128/jb.172.11.6596-6598.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. N., Hollingsworth R. I., Klein S., Signer E. R. The symbiotic defect of Rhizobium meliloti exopolysaccharide mutants is suppressed by lpsZ+, a gene involved in lipopolysaccharide biosynthesis. J Bacteriol. 1990 May;172(5):2622–2632. doi: 10.1128/jb.172.5.2622-2632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P., van Veldhuizen A., Fokkens R. H. Re-examination of cellular cyclic beta-1,2-glucans of Rhizobiaceae: distribution of ring sizes and degrees of glycerol-1-phosphate substitution. Antonie Van Leeuwenhoek. 1990 Apr;57(3):173–178. doi: 10.1007/BF00403952. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]





