Abstract
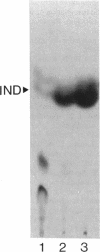
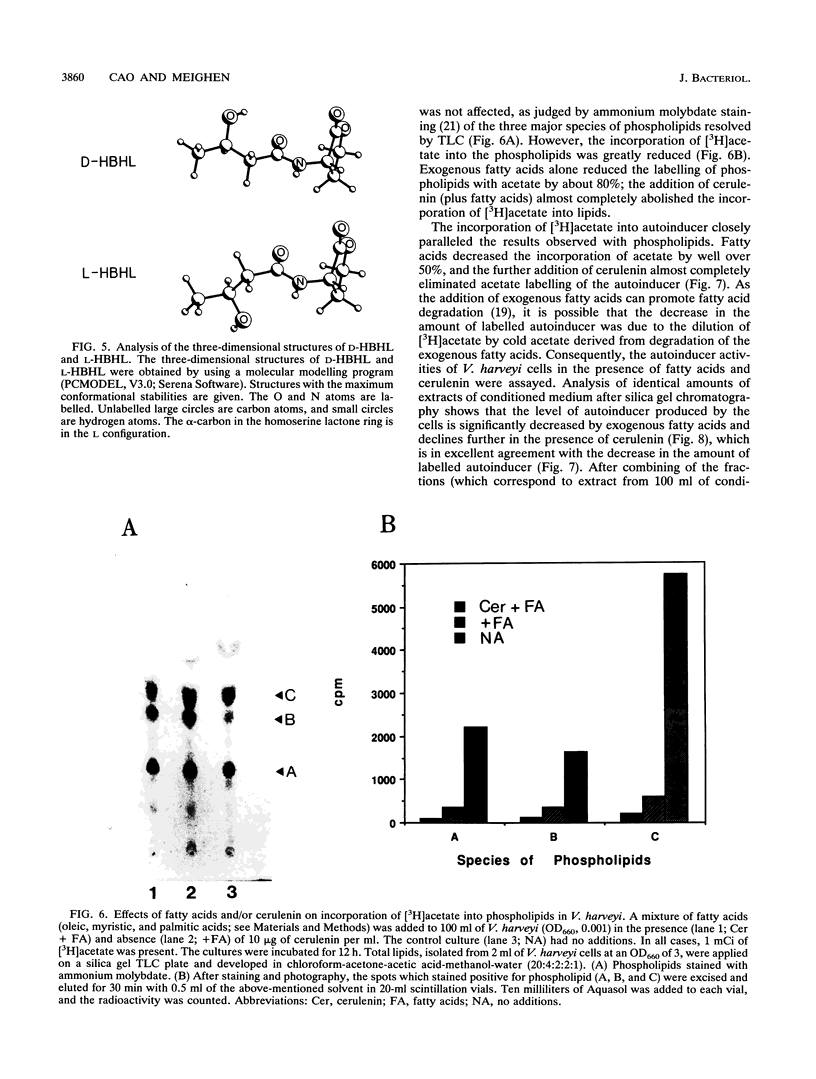
Knowledge of the pathway for synthesis of the autoinducer, N-(beta-hydroxybutyryl)-homoserine lactone (HBHL), controlling luminescence in Vibrio harveyi can provide important information concerning the relationship between the nutrition and physiology of the bacteria and the phenomenon of light emission. In this study, the D and L isomers of the autoinducer containing the stereoisomers of beta-hydroxybutyric acid were synthesized and characterized by proton nuclear magnetic resonance in the presence of a chiral shift reagent, a europium(III) derivative of Tris[3-(heptafluoropropyl-hydroxymethylene)-(+)-camphorato]. By using a newly isolated autoinducer mutant which responds to low physiological concentrations of the autoinducer, it could be shown that autoinducer activity was associated with D-HBHL and not L-HBHL. Blockage of fatty acid biosynthesis by the addition of fatty acids and/or the antibiotic cerulenin to the cells prevented synthesis of the autoinducer as measured by the loss of autoinducer activity and a decrease in the incorporation of labelled acetate into the partially purified autoinducer. These results indicate that fatty acid biosynthesis is necessary for light emission in luminescent bacteria because it controls formation of the lux autoinducer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- D'Agnolo G., Rosenfeld I. S., Awaya J., Omura S., Vagelos P. R. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973 Nov 29;326(2):155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- DiRusso C. C., Nunn W. D. Cloning and characterization of a gene (fadR) involved in regulation of fatty acid metabolism in Escherichia coli. J Bacteriol. 1985 Feb;161(2):583–588. doi: 10.1128/jb.161.2.583-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hughes K. T., Simons R. W., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: fadR superrepressor mutants are unable to utilize fatty acids as the sole carbon source. J Bacteriol. 1988 Apr;170(4):1666–1671. doi: 10.1128/jb.170.4.1666-1671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985 Sep;163(3):1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen E. A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991 Mar;55(1):123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Giffin K., Clark D., Cronan J. E., Jr Role for fadR in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1983 May;154(2):554–560. doi: 10.1128/jb.154.2.554-560.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu E. K., MacCoss M. Modification of the Dittmer-Lester reagent for the detection of phospholipid derivatives on thin-layer chromatograms. J Lipid Res. 1979 May;20(4):561–563. [PubMed] [Google Scholar]
- Vanden Boom T., Cronan J. E., Jr Genetics and regulation of bacterial lipid metabolism. Annu Rev Microbiol. 1989;43:317–343. doi: 10.1146/annurev.mi.43.100189.001533. [DOI] [PubMed] [Google Scholar]