Abstract
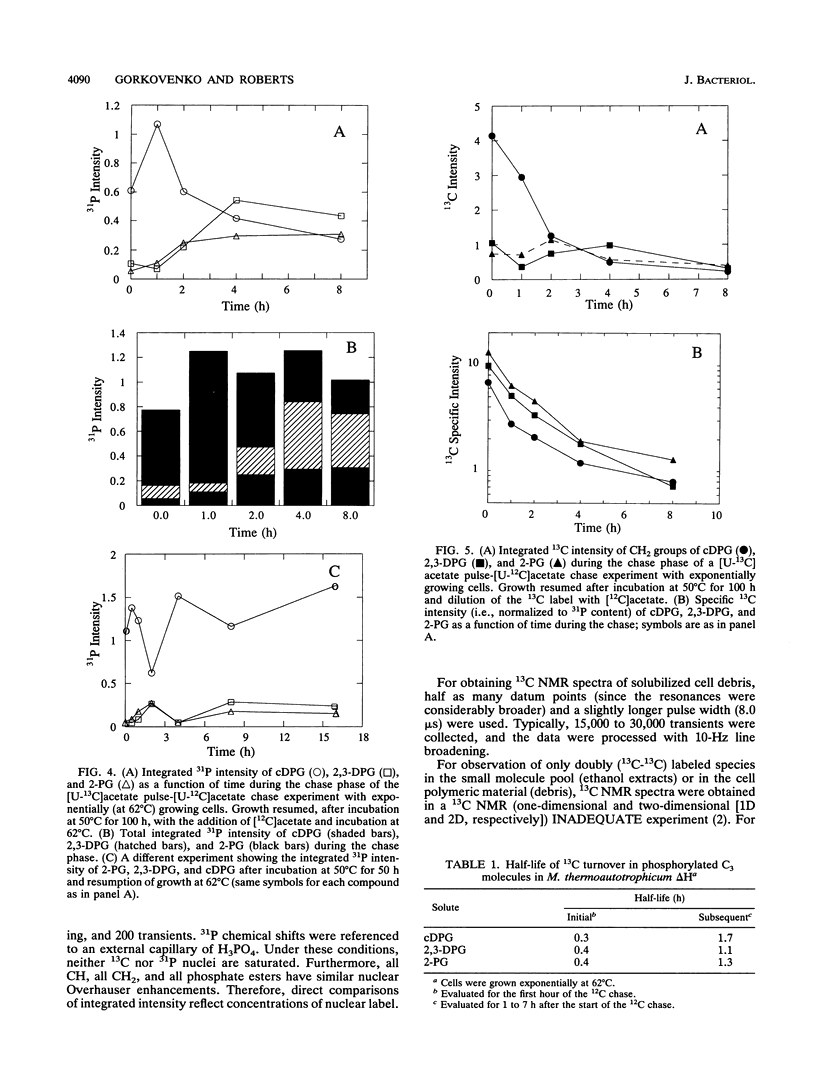
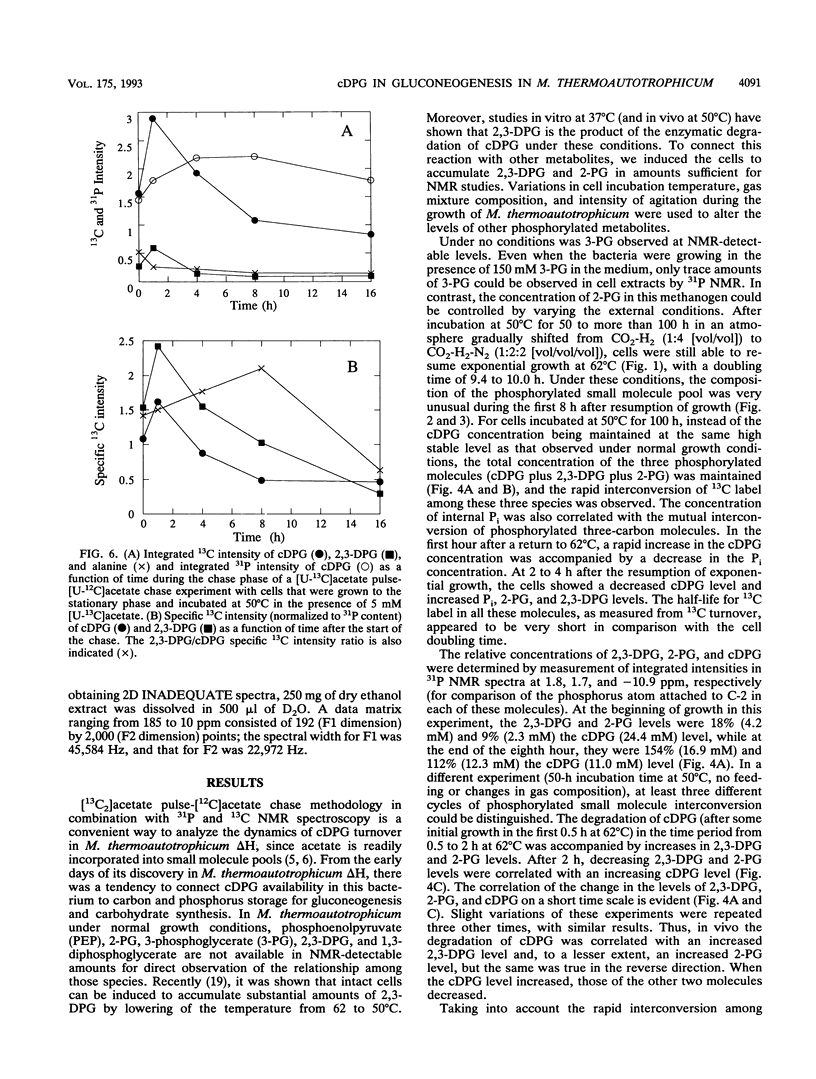
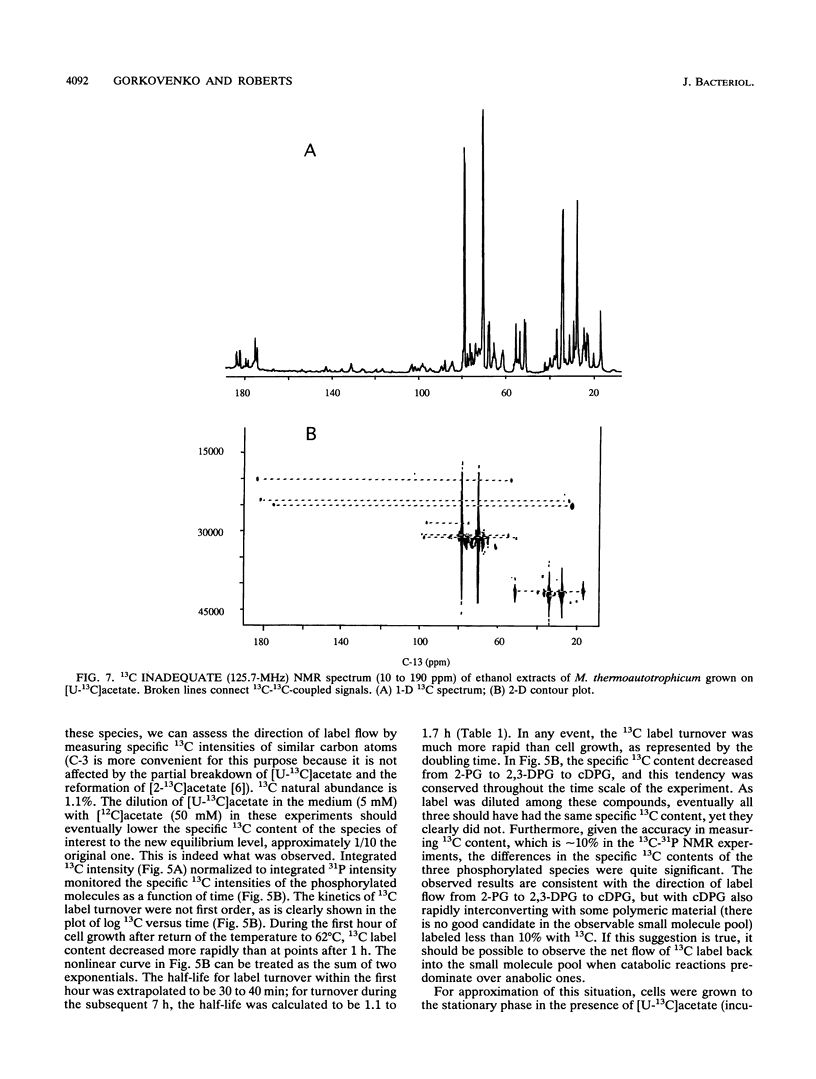
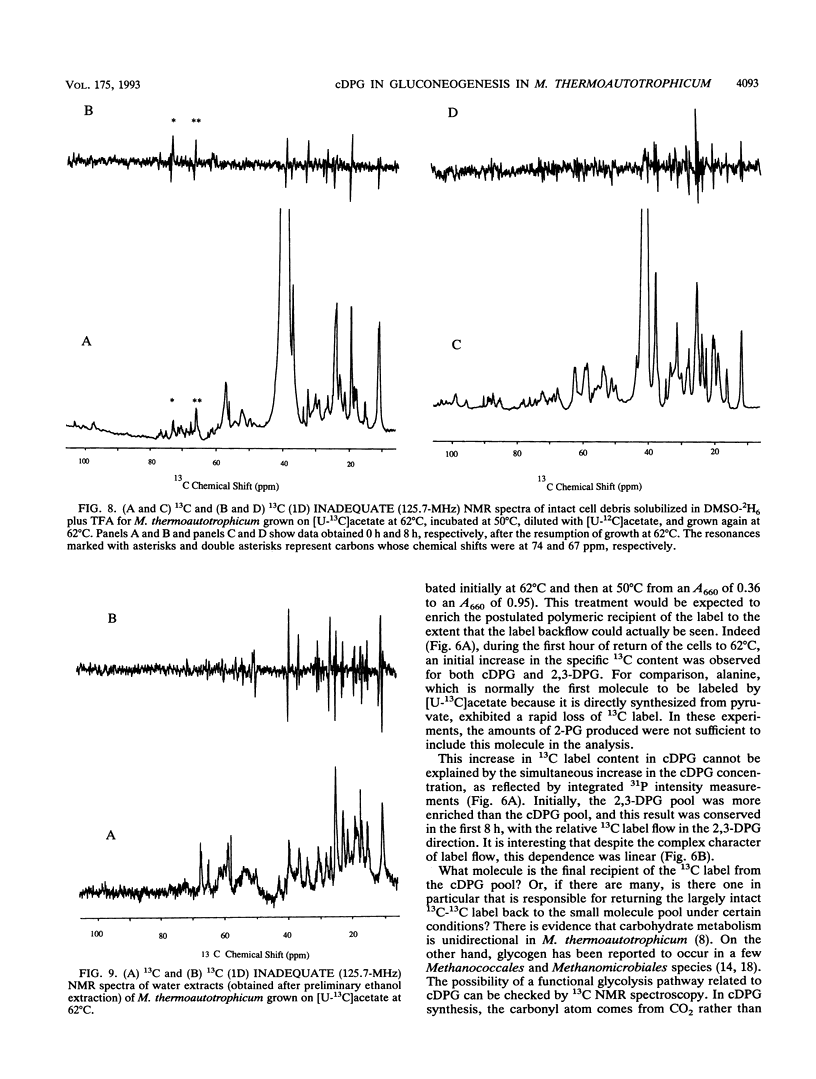
A unique compound, cyclic 2,3-diphosphoglycerate (cDPG), is the major soluble carbon and phosphorus solute in Methanobacterium thermoautotrophicum delta H under optimal conditions of cell growth. It is a component of an unusual branch in gluconeogenesis in these bacteria. [U-13C]acetate pulse-[12C]acetate chase methodology was used to observe the relationship between cDPG and other metabolites (2-phosphoglycerate and 2,3-diphosphoglycerate [2-PG and 2,3-DPG, respectively]) of this branch. It was demonstrated that cells could grow exponentially under conditions in which 2-PG and 2,3-DPG, rather than cDPG, were the major solutes. While the total concentration of these three phosphorylated molecules was maintained, rapid interconversion of 13C label among them was observed. Label flow from 2-PG to 2,3-DPG to cDPG to polymer is the usual direction in this pathway in exponentially growing cells, while the reverse reactions sometimes predominate in the stationary phase. Evidence of the presence of a polymeric compound in this pathway was provided by 13C nuclear magnetic resonance (one-dimensional and two-dimensional INADEQUATE) studies of solubilized cell debris.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Evans J. N., Raleigh D. P., Tolman C. J., Roberts M. F. 13C NMR spectroscopy of Methanobacterium thermoautotrophicum. Carbon fluxes and primary metabolic pathways. J Biol Chem. 1986 Dec 15;261(35):16323–16331. [PubMed] [Google Scholar]
- Evans J. N., Tolman C. J., Kanodia S., Roberts M. F. 2,3-Cyclopyrophosphoglycerate in methanogens: evidence by 13C NMR spectroscopy for a role in carbohydrate metabolism. Biochemistry. 1985 Oct 8;24(21):5693–5698. doi: 10.1021/bi00342a001. [DOI] [PubMed] [Google Scholar]
- Evans J. N., Tolman C. J., Roberts M. F. Indirect observation by 13C NMR spectroscopy of a novel CO2 fixation pathway in methanogens. Science. 1986 Jan 31;231(4737):488–491. doi: 10.1126/science.3079919. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanodia S., Roberts M. F. Methanophosphagen: Unique cyclic pyrophosphate isolated from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5217–5221. doi: 10.1073/pnas.80.17.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltjens J. T., Daniels L., Jannsen H. G., Borm P. J., Vogels G. D. A novel one-carbon carrier (carboxy-5,6,7,8-tetrahydromethanopterin) isolated from Methanobacterium thermoautotrophicum and derived from methanopterin. Eur J Biochem. 1983 Feb 15;130(3):545–552. doi: 10.1111/j.1432-1033.1983.tb07184.x. [DOI] [PubMed] [Google Scholar]
- Lehmacher A., Vogt A. B., Hensel R. Biosynthesis of cyclic 2,3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and cyclic 2,3-diphosphoglycerate synthetase from Methanothermus fervidus. FEBS Lett. 1990 Oct 15;272(1-2):94–98. doi: 10.1016/0014-5793(90)80456-s. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- Sastry M. V., Robertson D. E., Moynihan J. A., Roberts M. F. Enzymatic degradation of cyclic 2,3-diphosphoglycerate to 2,3-diphosphoglycerate in Methanobacterium thermoautotrophicum. Biochemistry. 1992 Mar 24;31(11):2926–2935. doi: 10.1021/bi00126a012. [DOI] [PubMed] [Google Scholar]
- Seely R. J., Fahrney D. E. A novel diphospho-P,P'-diester from Methanobacterium thermoautotrophicum. J Biol Chem. 1983 Sep 25;258(18):10835–10838. [PubMed] [Google Scholar]
- Tolman C. J., Kanodia S., Roberts M. F., Daniels L. 31P-NMR spectra of methanogens: 2,3-cyclopyrophosphoglycerate is detectable only in methanobacteria strains. Biochim Biophys Acta. 1986 May 29;886(3):345–352. doi: 10.1016/0167-4889(86)90169-2. [DOI] [PubMed] [Google Scholar]
- Turner J. D., Rouser G. Precise quantitative determination of human blood lipids by thin-layer and triethylaminoethylcellulose column chromatography. I. Erythrocyte lipids. Anal Biochem. 1970 Dec;38(2):423–436. doi: 10.1016/0003-2697(70)90467-7. [DOI] [PubMed] [Google Scholar]