Abstract
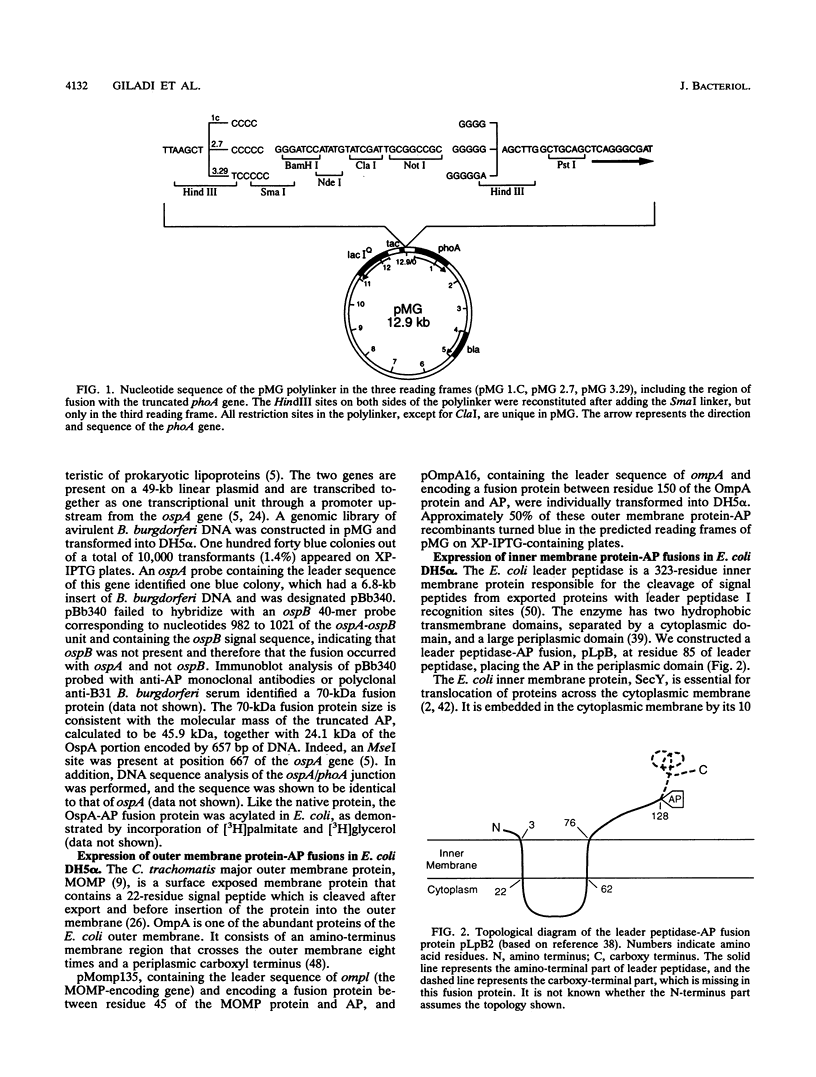
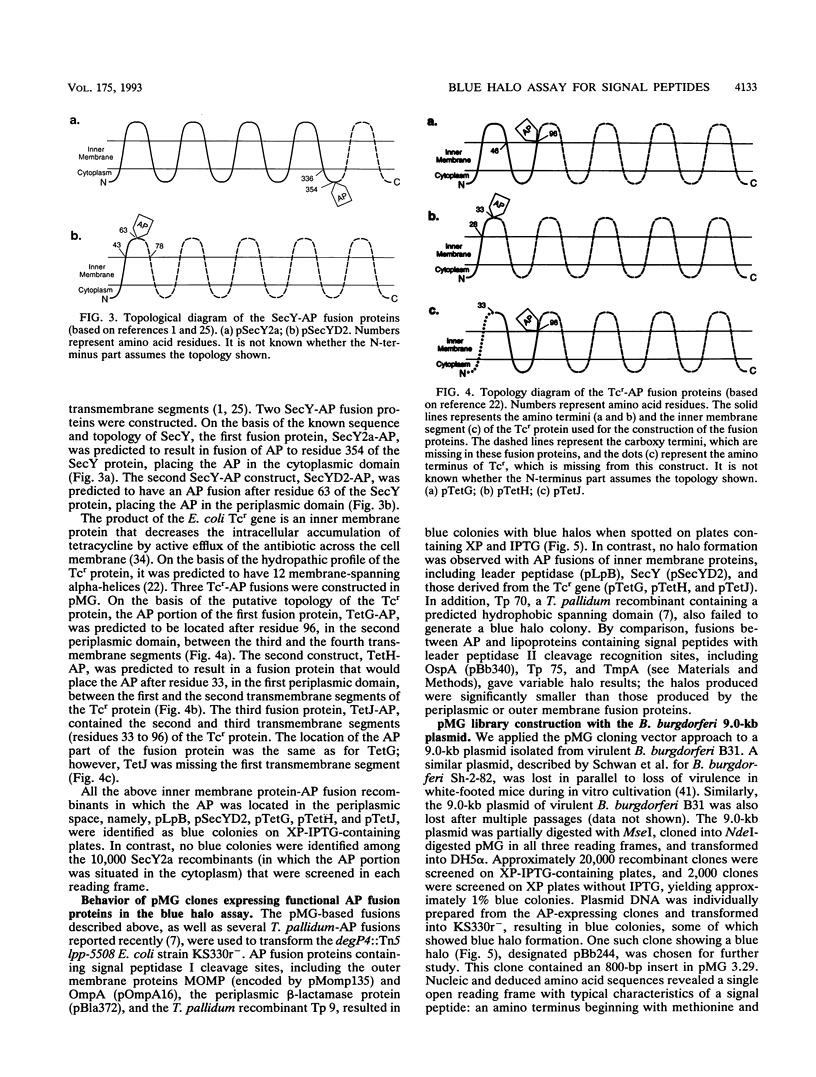
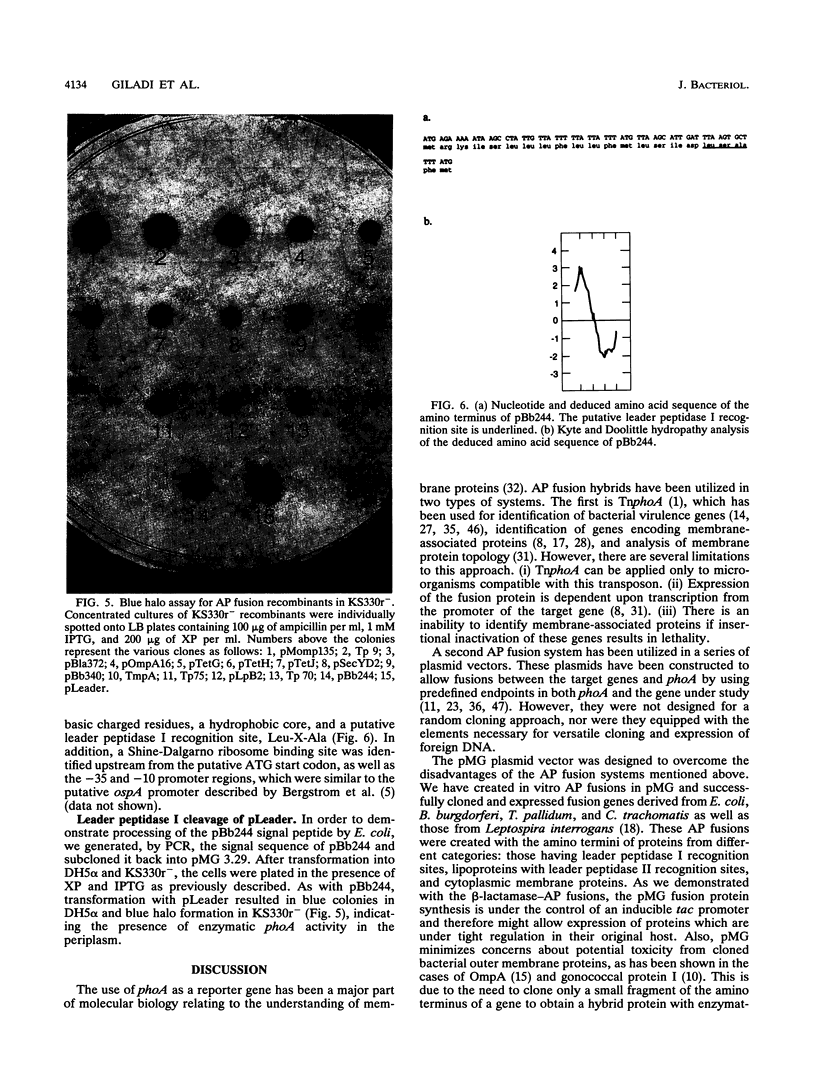
We have recently reported a phoA expression vector, termed pMG, which, like TnphoA, is useful in identifying genes encoding membrane-spanning sequences or signal peptides. This cloning system has been modified to facilitate the distinction of outer membrane and periplasmic alkaline phosphatase (AP) fusion proteins from inner membrane AP fusion proteins by transforming pMG recombinants into Escherichia coli KS330, the strain utilized in the "blue halo" assay first described by Strauch and Beckwith (Proc. Natl. Acad. Sci. USA 85:1576-1580, 1988). The lipoprotein mutation lpp-5508 of KS330 results in an outer membrane that is leaky to macromolecules, and its degP4 mutation greatly reduces periplasmic proteolytic degradation of AP fusion proteins. pMG AP fusions containing cleavable signal peptides, including the E. coli periplasmic protein beta-lactamase, the E. coli and Chlamydia trachomatis outer membrane proteins OmpA and MOMP, respectively, and Tp 9, a Treponema pallidum AP recombinant, diffused through the leaky outer membrane of KS330 and resulted in blue colonies with blue halos. In contrast, inner membrane AP fusions derived from E. coli proteins, including leader peptidase, SecY, and the tetracycline resistance gene product, as well as Tp 70, a T. pallidum AP recombinant which does not contain a signal peptide, resulted in blue colonies without blue halos. Lipoprotein-AP fusions, including the Borrelia burgdorferi OspA and T. pallidum Tp 75 and TmpA showed halo formation, although there was significantly less halo formation than that produced by either periplasmic or outer membrane AP fusions. In addition, we applied this approach to screen recombinants constructed from a 9.0-kb plasmid isolated from the B31 virulent strain of B. burgdorferi. One of the blue halo colonies identified produced an AP fusion protein which contained a signal peptide with a leader peptidase I cleavage recognition site. The pMG/KS330r- cloning and screening approach can identify genes encoding proteins with cleavable signal peptides and therefore can serve as a first step in the identification of genes encoding potential virulence factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao R., Crooke E., Shiba K., Wickner W., Ito K. The secY protein can act post-translationally to promote bacterial protein export. J Biol Chem. 1986 Sep 25;261(27):12907–12910. [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco D. R., Giladi M., Champion C. I., Haake D. A., Chikami G. K., Miller J. N., Lovett M. A. Identification of Treponema pallidum subspecies pallidum genes encoding signal peptides and membrane-spanning sequences using a novel alkaline phosphatase expression vector. Mol Microbiol. 1991 Oct;5(10):2405–2415. doi: 10.1111/j.1365-2958.1991.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Boquet P. L., Manoil C., Beckwith J. Use of TnphoA to detect genes for exported proteins in Escherichia coli: identification of the plasmid-encoded gene for a periplasmic acid phosphatase. J Bacteriol. 1987 Apr;169(4):1663–1669. doi: 10.1128/jb.169.4.1663-1669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Sparling P. F. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepuri V., Gennis R. B. The use of gene fusions to determine the topology of all of the subunits of the cytochrome o terminal oxidase complex of Escherichia coli. J Biol Chem. 1990 Aug 5;265(22):12978–12986. [PubMed] [Google Scholar]
- Crooke E., Guthrie B., Lecker S., Lill R., Wickner W. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell. 1988 Sep 23;54(7):1003–1011. doi: 10.1016/0092-8674(88)90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Starnbach M. N., Francis C. L., Stocker B. A., Chatfield S., Dougan G., Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988 Nov;2(6):757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Freudl R., Klose M., Henning U. Export and sorting of the Escherichia coli outer membrane protein OmpA. J Bioenerg Biomembr. 1990 Jun;22(3):441–449. doi: 10.1007/BF00763176. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Barondess J., Manoil C., Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J Mol Biol. 1987 May 20;195(2):289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
- Haake D. A., Champion C. I., Martinich C., Shang E. S., Blanco D. R., Miller J. N., Lovett M. A. Molecular cloning and sequence analysis of the gene encoding OmpL1, a transmembrane outer membrane protein of pathogenic Leptospira spp. J Bacteriol. 1993 Jul;175(13):4225–4234. doi: 10.1128/jb.175.13.4225-4234.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., Maiden M. C. Homologous sugar transport proteins in Escherichia coli and their relatives in both prokaryotes and eukaryotes. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):391–410. doi: 10.1098/rstb.1990.0020. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Wright A. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5107–5111. doi: 10.1073/pnas.82.15.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Structure, function, and biogenesis of SecY, an integral membrane protein involved in protein export. J Bioenerg Biomembr. 1990 Jun;22(3):353–367. doi: 10.1007/BF00763172. [DOI] [PubMed] [Google Scholar]
- Kaul R., Duncan M. J., Guest J., Wenman W. M. Expression of the Chlamydia trachomatis major outer membrane protein-encoding gene in Escherichia coli: role of the 3' end in mRNA stability. Gene. 1990 Mar 1;87(1):97–103. doi: 10.1016/0378-1119(90)90499-h. [DOI] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., McCune S., Walker G. C. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J Bacteriol. 1988 Sep;170(9):4257–4265. doi: 10.1128/jb.170.9.4257-4265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. S., Jackson E. N. Use of alkaline phosphatase fusions to study protein secretion in Bacillus subtilis. J Bacteriol. 1991 Apr;173(7):2278–2282. doi: 10.1128/jb.173.7.2278-2282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Millan J. L., Boyd D., Dalbey R., Wickner W., Beckwith J. Use of phoA fusions to study the topology of the Escherichia coli inner membrane protein leader peptidase. J Bacteriol. 1989 Oct;171(10):5536–5541. doi: 10.1128/jb.171.10.5536-5541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid G. P., Steigerwalt A. G., Johnson S. E., Barbour A. G., Steere A. C., Robinson I. M., Brenner D. J. DNA characterization of the spirochete that causes Lyme disease. J Clin Microbiol. 1984 Aug;20(2):155–158. doi: 10.1128/jcm.20.2.155-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A. R., Kaplan S. Construction, expression, and localization of a CycA::PhoA fusion protein in Rhodobacter sphaeroides and Escherichia coli. J Bacteriol. 1989 Nov;171(11):5830–5839. doi: 10.1128/jb.171.11.5830-5839.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Yu F., Inouye S., Inouye M. Lipoprotein-28, a cytoplasmic membrane lipoprotein from Escherichia coli. Cloning, DNA sequence, and expression of its gene. J Biol Chem. 1986 Feb 15;261(5):2284–2288. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]



