Abstract
Fibroblasts play a major role in tissue repair and remodeling. Their differentiation into myofibroblasts, marked by increased expression of smooth muscle–specific α-actin (α-SMA), is believed to be important in wound healing and fibrosis. We have recently described a role for MK2 in this phenotypic differentiation in culture. In this article, we demonstrate that MK2 also regulates myofibroblasts in vivo. Disruption of MK2 in mice prevented myofibroblast formation in a model of pulmonary fibrosis. However, MK2 disruption and consequent lack of myofibroblast formation exacerbated fibrosis rather than ameliorated it as previously postulated. When mice lacking MK2 (MK2−/−) were exposed to bleomycin, more collagen accumulated and more fibroblasts populated fibrotic regions in their lungs than in similarly treated wild-type mice. While there were many vimentin-positive cells in the bleomycin-treated MK2−/− mouse lungs, few α-SMA–positive cells were observed in these lungs compared with wild-type mouse lungs. siRNA against MK2 reduced α-SMA expression in wild-type mouse embryonic fibroblasts (MEF), consistent with its suppression in MK2−/− MEF. On the other hand expressing constitutively active MK2 in MK2−/− MEF significantly increased α-SMA expression. MK2−/−MEF proliferated at a faster rate and produced more collagen; however, they migrated at a slower rate than wild-type MEF. Overexpressing phosphomimicking HSP27, a target of MK2, did not reverse the effect of MK2 disruption on fibroblast migration. MK2 disruption did not affect Smad2 activation by transforming growth factor-β. Thus, MK2 appears to mediate myofibroblast differentiation, and inhibiting that differentiation might contribute to fibrosis rather than protect against it.
Keywords: actin, cytoskeleton, fibroblast, p38, idiopathic pulmonary fibrosis
CLINICAL RELEVANCE
Our findings implicate MK2 in the pathogenesis of pulmonary fibrosis. They further suggest that myofibroblasts might play a protective role in pulmonary fibrosis, in contrast to the commonly held view that they contribute to the pathogenesis of fibrosis.
Pulmonary fibrosis as a major component of interstitial lung disease is characterized by abnormal fibroblast proliferation and deposition of extracellular matrix proteins that remodel the normal pulmonary tissue structure and compromise its function. In some forms of interstitial lung disease, for example, idiopathic pulmonary fibrosis (IPF), the mechanism by which fibrosis arises remains poorly understood (1, 2). Differentiation of fibroblasts into myofibroblasts has long been believed to be an important event in many conditions such as wound repair and fibrosis. For example, it has been reported that myofibroblasts occur in areas of active fibrosis and are responsible for production and deposition of extracellular matrix (ECM) proteins in pulmonary fibrosis (3). We have recently described a key role for MK2 (mitogen-activated protein kinase–activated protein kinase 2 or MAPKAPK2) in the differentiation of mouse embryonic fibroblasts into myofibroblasts (4). We now demonstrate that MK2 is also necessary for myofibroblast formation in vivo in a model of pulmonary fibrosis. Furthermore, our results indicate that MK2 disruption leads to more severe pulmonary fibrosis in response to bleomycin treatment, suggesting that it might be a target in pulmonary and other types of fibrosis.
Fibrosis arises in several tissues in response to inflammation and chemical injury, or idiopathically, leading to accumulation of fibroblasts that deposit ECM proteins. Such processes are part of normal wound healing, and are often reversible by apoptosis of accumulating fibroblasts and resorption of ECM proteins. However, in fibrosis the proliferation of fibroblasts and ECM deposits appear excessive and dysregulated, resulting in the gradual displacement of normal tissue by fibrotic foci that disrupt tissue function. Studies aimed at elucidating the mechanisms of fibrosis implicated certain cytokines in animal models as well as in human patients. In the lungs of patients with IPF, transforming growth factor (TGF)-β (5), IL-1β, and TNF-α (6, 7) were reported to be elevated. Indeed TGF-β is believed to play a pivotal role in fibrosis, and its overepxression in mice is sufficient to cause pulmonary fibrosis independent of injury and inflammation (8, 9). TGF-β is a cytokine that can exert many actions and has been implicated in diseases ranging from cancer to pulmonary hypertension. One action of TGF-β that has been of interest in fibrosis research is its causing fibroblasts to differentiate into myofibroblasts, which are known to accumulate in sites of fibrosis (3, 10–13).
Myofibroblasts, which are believed to play a role in wound contraction, are more contractile than fibroblasts due to increased expression of the smooth muscle–specific proteins, such as α-SMA (for review, see Ref. 14). Studies into the mechanism by which myofibroblast differentiation occurs focused on signaling pathways activated by TGF-β in fibroblasts. TGF-β is known to signal through Smad proteins (for review, see Ref. 15), but can also activate Ras and Erk (16, 17), Rho GTPase and JNK (18), as well as p38 MAP kinase (19). Inhibition of p38 has been reported to reduce pulmonary (20, 21) and renal (22) fibrosis in animal models. We have recently reported that MK2, a kinase which is a substrate for p38, plays an important role in the differentiation of fibroblasts into myofibroblasts (4). Our findings demonstrated that disrupting the expression of MK2 in mouse embryonic fibroblasts causes these cells to express significantly less α-SMA. Furthermore, cells that lacked MK2 did not increase α-SMA expression in response to treatment with TGF-β as did wild-type fibroblasts (4). Thus MK2 appeared to be necessary for myofibroblast differentiation.
We launched this current study to examine whether the disruption of MK2 in knockout mice will also reduce myofibroblast differentiation in vivo and whether it will affect the development of fibrosis. Using bleomycin to model pulmonary fibrosis, we demonstrate that MK2 is indeed important for myofibroblast formation in vivo. Furthermore, absence of MK2 and lack of myofibroblast formation resulted in more severe fibrosis. These findings suggest that myofibroblast formation might be part of the repair phase of fibrosis rather than of active damage, and that MK2 activity is necessary for that process to occur.
MATERIALS AND METHODS
Materials and Reagents
Media and supplements (Dulbecco's modified Eagle's medium [DMEM]), fetal bovine serum (FBS), penicillin G potassium, streptomycin, fungizone, and glutamine were purchased from Invitrogen (Carlsbad, CA). TGF-β was purchased from R&D Systems (Minneapolis, MN) and activated before use according to manufacturer's instructions. SB431542 and all other reagents and drugs were obtained from Sigma (St. Louis, MO). On the day of the experiments, all drugs were diluted in serum-free medium.
Animals
Mice that lack MK2 (MK2−/−) were generated as described earlier (23). In brief, a neo selection cassette, which contains translational stop codons in all three reading frames, was inserted into the Sac I site located in the exon that encodes subdomains V and VI of MK2. MK2−/− mice were then backcrossed into C57BL/6J mice, and lack of expression of MK2 was verified by PCR and immunoblotting as described earlier (4). Wild-type C57BL/6J from Charles River Laboratories (Wilmington, MA) were used as controls for the described experiments. These mice were anesthetized with xylozine-ketamine, and then saline or bleomycin (3 U/kg in 50 μl) were introduced into their lungs by insufflation.
Cell Culture
Immortalized mouse embryonic fibroblasts (MEF) from wild-type and MK2−/− knockout mice were prepared as described earlier (23). Primary embryonic fibroblasts from C57BL/6J wild-type or MK2−/− mice were co-transfected with pSV40Tag encoding the SV40 large T antigen and the pREP8 plasmid, followed by selection and expansion of histidinol-resistant colonies. MEF cells were maintained in DMEM containing 10% FBS, penicillin, streptomycin, fungizone, and glutamine at 37°C in humidified air containing 5% CO2. MEF were passaged in 0.25% trypsin–0.02% EDTA solution, and 1 day before the experiments cells were maintained in serum-free or 1% serum–containing media.
For rescue experiments, MK2−/− MEF were transfected with pcDNA3-MK2EE, in which the residues T205E and T317E were mutated to act as constitutively active MK2 (caMK2) as we described earlier (24). The pcDNA3-MK2EE vector was co-transfected with pMEpuro (a selection vector that confers resistance to puromycin to eukaryotic cells). For studying the effects of HSP27 phosphorylation, wild-type and MK2−/− MEF were transfected with the pcDNA3-HSP27PM (25) to express HSP27 in which the residues, S15D, S78D, and S82D were mutated to mimic phosphorylated HSP27 (pmHSP27). The pmHSP27 vector was introduced alone in the case of wild-type MEF, and co-transfected with pMEpuro in the case of MK2−/− MEF. The vectors were introduced (5 μg of pmHSP27 or caMK2, and 0.5 μg pMEpuro) into MEF using lipofectamine. Stable transfected wild-type MEF cell lines were obtained by selection with geneticin, and resistant colonies were isolated, expanded, and then screened for pmHSP27 or MK2 by immunoblotting of cell lysates with anti-HSP27 or anti MK2 antibodies. As MK2−/− MEF were already resistant to geneticin, stable transfectants produced by co-transfection with pMEpuro were selected with puromycin, and resistant clones were isolated and expanded as above.
For proliferation assays, cells were seeded in 12-well dishes and cultured for 24, 48, and 72 hours. Counting was performed after trypsinizing the cells using a Coulter counter apparatus according to manufacturer's instructions.
For migration assays, cells were plated in 35-mm dishes. After 24 hours the monolayer was scratched with a sterile tip to make the wounding gap (time 0 h). Cells were cultured for another 24 hours in 10% serum media and then fixed with 4% formaldehyde for 10 minutes. Four pictures were randomly taken from duplicate dishes and wound closure was compared with time 0 h to assess cell migration.
Collagen Assay
After 14 days of exposure to bleomycin or saline, lungs were removed and snap frozen. Next, lungs were assayed for acid-soluble collagen using the Sircol Assay (Biocolor Ltd, Newtownabbey, UK). In brief, lungs were minced into small cubes and incubated overnight in 10 vols of 0.5 M acetic acid. Next, homogenates were incubated with an aliquot of Sircol Dye reagent (Sirus Red in picric acid) for 30 minutes. The collagen-dye complex was then pelleted by centrifugation at 10,000 × g for 10 minutes. The dye was next extracted with 0.5 M NaOH, and the absorbance of samples along with known collagen standards was measured at 540 nm in a Tecan Spectrafluor plate reader. The same assay was used to assay collagen in conditioned media from MEF cells (without the acid step) and in cell lysates (scraped into 0.5 M acetic acid).
Histochemistry and Immunohistochemistry
Lungs were instilled intratracheally with 4% formaldehyde at 21 cm H2O pressure. The tissue blocks were then embedded in paraffin, and 4-μm sections were cut for staining. Gomori's Trichrome staining was used to highlight collagen deposition. The Gomori's Trichrome staining was used to assign a fibrosis score as follows. Three investigators were asked to blindly score three sections from each animal (seven saline-treated wild-type, eight bleomycin-treated wild-type, four saline-treated MK2−/−, and eight bleomycin-treated MK2−/− mice). Each slide was given a value as 0, 1, or 2 according to the area and degree of target staining excluding airways and blood vessels, and the scores were pooled and averaged. Other sections were immunostained with antibodies against α-SMA (1A4; Biogenex, San Ramon, CA) and vimentin (VIM 3B4; Ventana, Tucson, AZ) using an automated immunostainer (Ventana) according to manufacturer's instructions. An IgG control (mouse IgG; Ventana) was included to control for nonspecific staining. The immunostainer uses biotinylated secondary antibodies and horseradish peroxidase complexed by avidin, followed by diaminobenzidine for colorimetric detection (Ventana). Three sections from two animals/treatment group were processed for immunolabeling simultaneously to control for staining variability. The area and degree of labeling, excluding airways and blood vessels, were used to rank the sections blindly by three investigators. The assigned scores were pooled and averaged as with the Trichrome staining.
siRNA Transfection
Eighty to ninety percent confluent cells were transfected with MK2 siRNA (Cat # 16704; Ambion, Austin, TX) and negative control siRNA (Cat # 4611; Ambion) with Lipofectamine 2000 (Invitrogen) at indicated concentrations for 30 hours according to the manufacturer's instructions. Western blotting was then performed to test the silencing effect of siRNA on the expression levels of target proteins.
SDS-PAGE and Immunoblotting
Cell lysates were assayed for protein using the Bradford protein assay (26) and then diluted with 2× Laemmli loading buffer for SDS-PAGE (27). Equal amounts of protein were then loaded in 4 to 20% Tris/glycine gels, and electrophoresed for 90 minutes at 125 V constant voltage. Next, the gel was blotted onto an Immobilon-P membrane by electrophoretic transfer at 25 V constant voltage overnight. The membrane was then washed, blocked with 5% milk, and probed with antibodies against MK2, p38, and phospho p38 (Cell Signaling, Beverly, MA), α-SMA (1A4; Sigma), pan-actin (C-2; Santa Cruz, Santa Cruz, CA), smad2/3(18, BD-Transduction Laboratories, San Jose, CA), and phospho-smad2 (138D4; Cell Signaling). Appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL), and a chemiluminescent substrate (SuperSignal; Pierce) were used according to the manufacturer's instructions to visualize immunoreactive bands.
Statistical Analysis
Data are presented as means ± SD. Statistical differences were determined by either Student's t test for comparison of two sample means or ANOVA for comparison of more than two sample means followed by Holm-Sidak post hoc testing for multiple comparisons between two sample means. Holm-Sidak test considers the rank of the P values of each comparison and the number of comparisons in calculating statistical significance. Statistical analysis was performed using SigmaStat (Systat, San Jose, CA). P < 0.05 was considered statistically significant.
RESULTS
MK2−/− Mice Are More Susceptible to Fibrosis than Wild-Type Mice
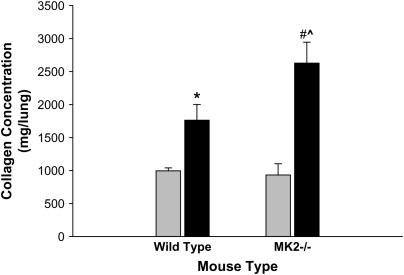
We have recently described that embryonic fibroblasts (MEF) isolated from MK2−/− mice expressed less α-SMA than their wild-type counterparts either at baseline or in response to TGF-β (4). In continuation of this project we attempted to ascertain the relevance of these findings to the whole animal. Thus we assessed the development of pulmonary fibrosis in MK2−/− mice compared with wild-type mice. Animals from each group were exposed to either saline or bleomycin, a chemotherapeutic associated with pulmonary fibrosis in human patients that has been used extensively to model pulmonary fibrosis in animals. After 2 weeks of exposure to a single dose of saline or bleomycin, mice were evaluated for the amount of acid-soluble collagen in their lungs. An increase in collagen deposition is associated with fibrosis. As shown in Figure 1, treatment with bleomycin caused a significant increase in acid-soluble lung collagen content in both wild-type and MK2−/− mice. However, the increase in collagen in response to bleomycin was significantly higher in MK2−/− mice (180%) than in wild-type mice (77%). These data suggested that MK2−/− mice might be more susceptible to fibrosis than wild-type mice.
Figure 1.
MK2−/− lungs produce more collagen in response to bleomycin treatment than wild-type lungs. Bleomycin caused an increase in acid-soluble collagen deposition in both wild-type and MK2−/− lungs. However, the collagen deposition was significantly higher in MK2−/− bleomycin-treated lungs than in wild-type bleomycin-treated lungs. * Statistically significant difference from saline-treated wild-type mean, # statistically significant difference from saline-treated MK2−/− mean, ^ statistically significant difference from bleomycin-treated wild-type mean. P < 0.05 in ANOVA and Holm-Sidak post hoc analysis. Shaded bars, saline; solid bars, bleomycin.
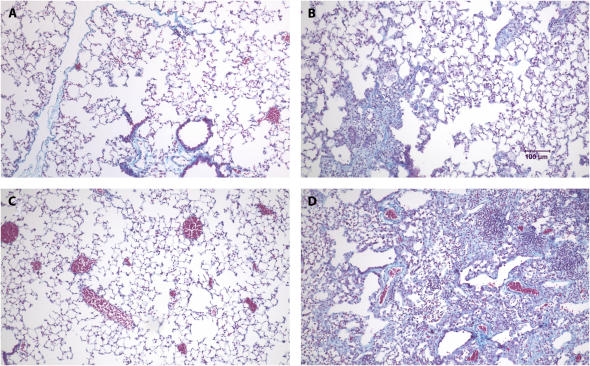
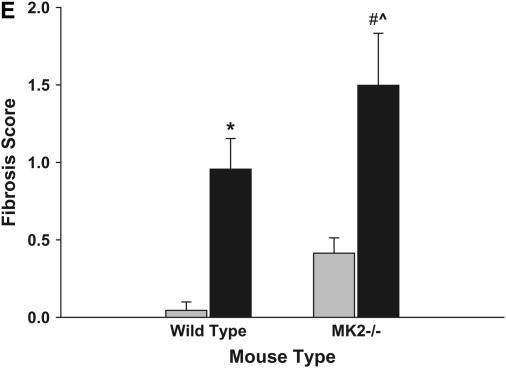
To confirm the findings of the collagen assay, we examined lungs from the mice treated as described above histochemically. After perfusion and fixation, lung sections were stained with Gomori's Trichrome stain, which stains collagen. As shown in Figures 2A–2D, the bleomycin-treated animals had significant cellularity in regions that should correspond to airspaces. The Trichrome stain also revealed increased collagen deposition (blue) in interstitial spaces of bleomycin-treated mice compared with saline-treated mice (Figures 2A–2D). Furthermore, the bleomycin-treated MK2−/− mice developed more severe fibrosis compared with the wild-type mice. To quantify the fibrosis in these mice, we ranked several Trichrome-stained sections from differently treated mice as described in Materials and Methods. Figure 2E shows the average and standard deviations of the pooled fibrosis scores. Both wild-type and MK2−/− bleomycin-treated mice had significantly higher fibrosis scores than the saline-treated corresponding mice. Moreover, there was a statistically significant difference between the wild-type and MK2−/− bleomycin-treated mice, indicating that MK2−/− mice developed more severe pulmonary fibrosis than wild-type mice.
Figure 2.
MK2−/− mice develop more severe fibrosis in response to bleomycin than wild-type mice. Treatment of wild-type mice with bleomycin (B) caused significant cellularity and collagen deposition (blue staining) relative to saline-treated wild-type mice (A), as revealed by Gomori's Trichrome stain. MK2−/− mice exhibited more severe cellularity and collagen deposition in response to bleomycin (D), compared with saline-treated MK2−/− mice (C) and bleomycin-treated wild-type mice (B). Several slides were scored as described in Materials and Methods. Average scores demonstrate that bleomycin-treated MK2−/− mice develop more severe fibrosis than bleomycin-treated wild-type mice (E). Shaded bars, saline; solid bars, bleomycin. * Statistically significant difference from saline-treated wild-type mean, # statistically significant difference from saline-treated MK2−/− mean, ^ statistically significant difference from bleomycin-treated wild-type mean. P < 0.05 in ANOVA and Holm-Sidak post hoc analysis.
Disruption of MK2 Leads to α-SMA Suppression, and Overexpression of MK2 Rescues Expression of α-SMA in MK2−/− Fibroblasts
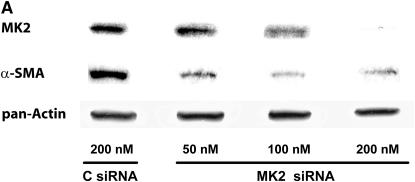
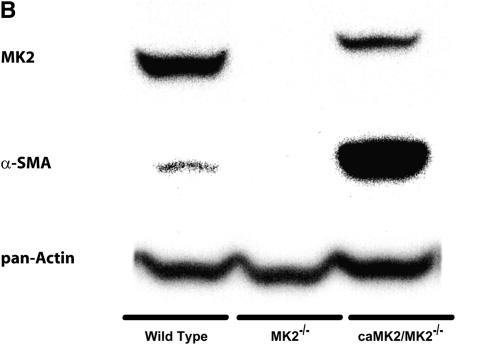
We have recently reported that MEF from MK2−/− mice express much less α-SMA than MEF from wild-type mice (4). Since different populations of fibroblasts have been reported to produce different amounts of α-SMA, we wished to eliminate the possibility that our findings were due to isolation of different populations of MEF from MK2−/− and wild-type mice. Furthermore, we wanted to test if the observed reduction in α-SMA in MK2−/− MEF was due to a developmental effect in MK2−/− mice. Thus we used siRNA against MK2 to suppress the expression of MK2 in wild-type MEF. As shown in Figure 3, MK2 siRNA transfection caused a significant reduction in the level of MK2 expressed in wild-type MEF compared with negative control siRNA-transfected MEF. When these cells were probed for the expression of α-SMA, we observed that the MK2 siRNA transfecteion also inhibited the expression of α-SMA (Figure 3A). To further test the causal relationship between MK2 activity and α-SMA expression, we stably transfected MK2−/− MEF with a constitutively active form of MK2 (caMK2). As shown in Figure 3B, overexpression of caMK2 resulted in a significant increase in α-SMA expression. These findings indicate that MK2 plays an important role in myofibroblast differentiation regardless of fibroblast population or developmental effects.
Figure 3.
Knocking down MK2 reduces α-SMA expression. Expression of constitutively active MK2 in MK2−/− MEF restores α-SMA expression. Transfection of wild-type MEF with siRNA against MK2 reduced the level of MK2 and α-SMA relative to negative control siRNA (C siRNA) transfection in a dose-dependent manner (A). MK2 siRNA transfection had no effect on total actin expression (A). On the other hand, transfection of MK2−/− MEF with a constitutively active form of MK2 (caMK2) caused a significant increase in α-SMA expression (B).
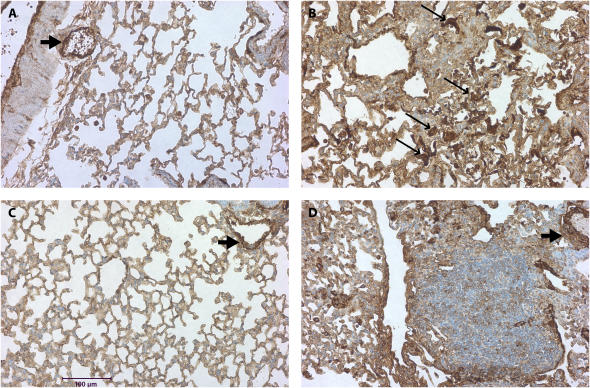
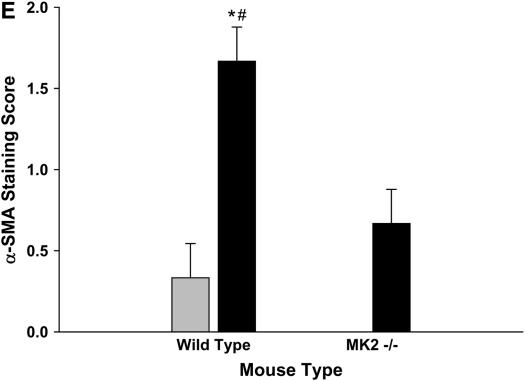
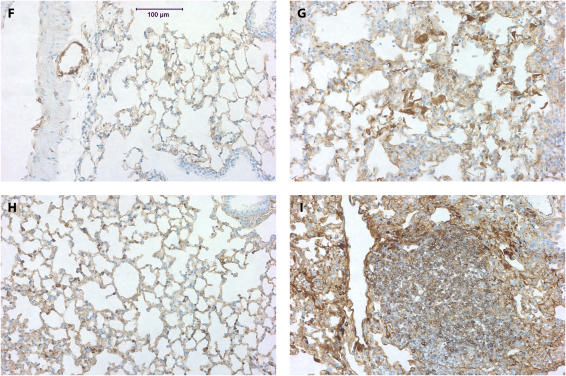
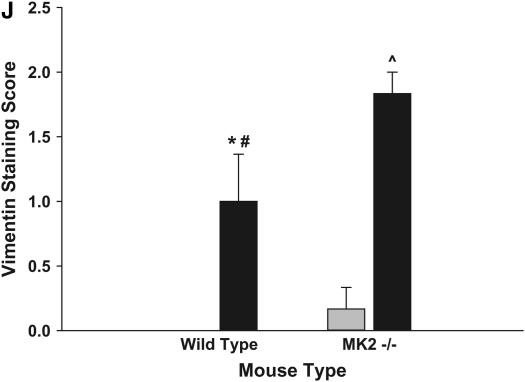
We next evaluated the expression of α-SMA in the lungs of MK2−/− and wild-type mice. Thus we stained lung sections from mice treated with saline or bleomycin as described above with an antibody against α-SMA. Smooth muscle cells in wild-type and MK2−/− lungs both stained positively for α-SMA in the vasculature and airways independently of the treatment (Figures 4A–4E), suggesting that MK2 disruption has no effect on α-SMA expression in smooth muscle cells. However, in the bleomycin-treated mice, a different staining pattern for fibroblasts emerged. In lung sections from wild-type bleomycin-treated mice, there were many cells in areas of fibrosis that stained positively for α-SMA actin (Figure 4B), consistent with the presence of myofibroblasts in these lesions. However, areas of extensive fibrosis in the MK2−/− bleomycin-treated mice contained minimal staining for α-SMA (Figure 4D). We attempted to quantify differences in α-SMA staining by having three investigators blindly rank several slides for the degree of α-SMA labeling in fibrotic areas. As shown in Figure 4E, MK2−/− sections contained significantly less α-SMA–positive fibroblasts than wild-type sections. Furthermore, the number of α-SMA–positive cells populating areas of fibrosis in bleomycin-treated MK2−/− sections were not significantly different from either wild-type or MK2−/− saline-treated sections. We confirmed the presence of fibroblasts in these areas using an antibody against vimentin, which revealed many vimentin-positive cells in areas of fibrosis in both wild-type and MK2−/− belomycin-treated mice (Figure 4F-I). Indeed scoring of vimentin slides in the manner we followed with α-SMA staining revealed more vimentin staining in bleomycin-treated MK2−/− sections than in bleomycin-treated wild-type sections (Figure 4J). These findings indicate that extensive pulmonary fibrosis can occur at a more severe degree without correlating with a higher number of α-SMA–positive fibroblasts. Since α-SMA is one of the most commonly used markers for myofibroblasts, a reexamination of its role in defining myofibroblasts or the role of myofibroblasts in fibrosis might be warranted.
Figure 4.
Fibrotic lesions in MK2−/− mice contain few α-SMA–positive cells, although they contain many vimentin-positive cells, compared with wild-type fibrotic lesions: Lung sections were stained with an anti–α-SMA (A–D) or anti-vimentin (F–I) antibodies. Both wild-type and MK2−/− sections expressed α-SMA in smooth muscle cells (A, C, D, thick arrows). However, α-SMA–positive cells (thin arrows, B) were much more prominent in fibrotic regions of bleomycin-treated wild-type lung sections. Both wild-type and MK2−/− sections expressed vimentin in fibrotic lesions (G, I). However, vimentin-positive cells were much more prominent in fibrotic regions of bleomycin-treated MK2−/− lung sections, reflecting a higher degree of fibrosis (G, I). (A, F) Saline-treated wild-type, (B, G) bleomycin-treated wild-type, (C, H) saline-treated MK2−/−, (D, I) bleomycin-treated MK2−/−. Blind scoring of several anti–α-SMA–labeled slides from each treatment as described in Materials and Methods demonstrates that, excluding smooth muscle cells in airways and blood vessels, bleomycin-treated MK2−/− lung sections contain significantly less α-SMA–positive cells than bleomycin-treated wild-type lung sections (E). On the other hand, similar scoring of anti-vimentin–labeled slides demonstrates that, bleomycin-treated MK2−/− lung sections contain significantly more vimentin-positive cells than bleomycin-treated wild-type lung sections (J). Shaded bars, saline; solid bars, bleomycin. * Statistically significant difference from saline-treated wild-type mean, # statistically significant difference from bleomycin-treated MK2−/− mean, ^ statistically significant difference from saline-treated MK2−/− mean. P < 0.05 in ANOVA and Holm-Sidak post hoc analysis.
Embryonic Fibroblasts from MK2−/− Mice Exhibit a Higher Proliferative Rate and Secrete More Collagen than Wild-Type Fibroblasts
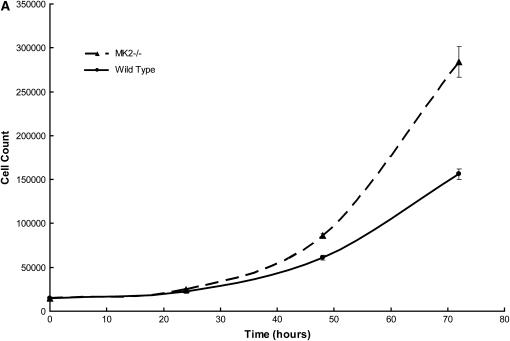
Since both MEF and whole animals exhibit the same characteristic inhibition of α-SMA in response to MK2 disruption, we investigated other characteristics of fibroblasts in culture that might explain the observations we made in bleomycin-treated mice. When seeded at equal density, MEF from MK2−/− mice grew at a faster rate than their wild-type counterparts. This effect was observed at 10% serum and in the absence of serum, such that MK2−/− were more tolerant to serum deprivation. As shown in Figure 5A, MK2−/− MEF cell count increases faster than wild-type MEF. Such increased proliferation rate might explain the excessive cellularity observed in fibrotic foci in bleomycin-treated MK2−/− mouse lungs.
Figure 5.
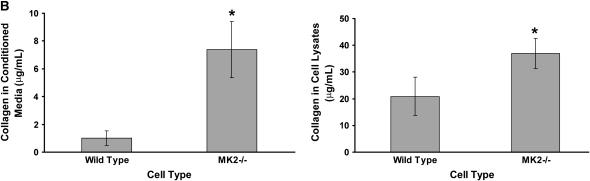
MK2−/− MEF proliferate faster and produce more collagen than wild-type MEF. When wild-type and MK2−/− MEF were seeded at equal density, and counted after 24, 48, and 72 h, MK2−/− MEF cell count increased faster than wild-type MEF cell count (A). MEF from wild-type and MK2−/− mice were seeded at equal density. After 24 hours, collagen was assayed as described in Materials and Methods in conditioned media, as well as in attached cells. MK2−/− MEF produced more collagen than their wild-type counterparts (B). *Statistically significant difference from wild-type mean, P < 0.05 in t test.
We next assayed collagen in wild-type and MK2−/− MEF after seeding at equal density and maintaining in 1% serum for 24 hours. As shown in Figure 5B, MK2−/− fibroblasts produced more collagen than their corresponding wild-type fibroblasts. These data are consistent with the increased collagen deposition we observed in the lungs of bleomycin-treated MK2−/− mice relative to wild-type mice (Figure 1).
MK2−/− Fibroblasts Are Defective in Migratory Activity and the Defect Is Not Due to Lack of Phosphorylated HSP27
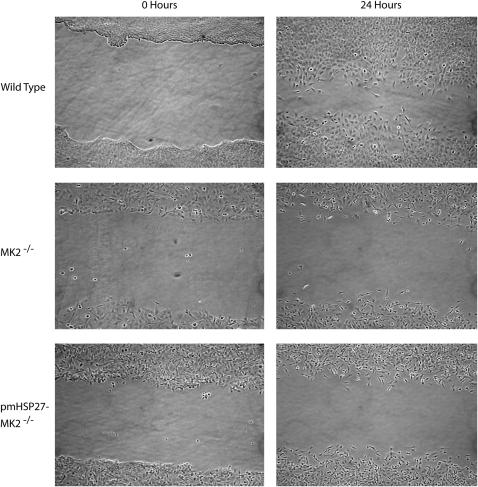
We have already established that MEF from MK2 mice lack α-SMA and linked that causally to MK2 expression. MK2−/− MEF have been described to migrate more slowly than their wild-type counterparts (28). We have assayed the ability of MEF to repopulate a wound made by a sterile pipette in a tissue culture dish. As seen in Figure 6, wild-type MEF repopulated the wounded area much faster than the MK2−/− cells. As MK2 is known to alter the actin cytoskeleton through its direct action on the small heat shock protein HSP27, we also generated stably transfected phosphomimicking (pm) HSP27 MK2−/− MEF. We have previously demonstrated that expressing this construct stably in endothelial cells causes an increase in actin stress fibers, which is believed to represent a more contractile phenotype (24). While transfected MK2−/− MEF expressed pm HSP27, they were still defective in migration compared with wild-type cells (Figure 6). These results indicate that MK2 affects migration of fibroblasts, not through its effect on HSP27 phosphorylation, but possibly through a direct effect on α-SMA expression.
Figure 6.
MK2−/− MEF are slower than wild-type MEF in repopulating a wounded part of the culture dish. Cells were scraped off subconfluent MEF culture dishes of equal density using a sterile pipette tip. After 24 hours, more wild-type MEF than MK2−/− MEF were observed in the wounded area. Overexpressing phosphomimicking pmHSP27 in MK2−/− cells did not reverse the defective migratory properties of MK2−/− MEF.
Disruption of MK2 Does Not Affect Smad Signaling, Which Appears to Be Upstream of p38
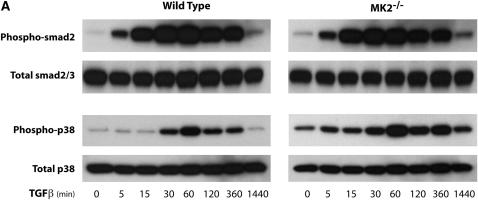
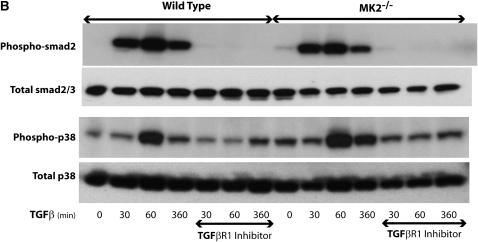
Fibroblast proliferation and α-SMA expression have been linked to factors such as TGF-β, which is known to exert many of its effects through Smad signaling. Thus we wished to examine this signaling pathway in MK2−/− versus wild-type MEF. After treatment with TGF-β (1 ng/ml) for different times, we assayed Smad2 phosphorylation. As shown in Figure 7A, Smad2 phosphorylation is activated by TGF-β similarly in wild-type or MK2−/− MEF, indicating that MK2 is not upstream of Smad2 in TGF-β–activated signaling. We also examined the activation of p38, which is an upstream activator of MK2, by TGF-β in both cell types, and demonstrated that it is indeed activated, albeit at a later time-course than Smad2 (Figure 7A). The fact that p38 becomes activated in MK2−/− cells is consistent with its being upstream of MK2. When wild-type or MK2−/− fibroblasts were preincubated with an inhibitor of TGF-β Type I receptor kinase SB431542 (10 μM), Smad2 activation by TGF-β peaking at 60 minutes was inhibited in both cell types. Furthermore, inhibiting Smad2 activation by SB431542 also inhibited p38 activation (Figure 7B). These findings suggest that Smad2 signals upstream of MK2 and that the effects of MK2 on fibroblast physiology and fibrosis are not due to altered downstream Smad signaling.
Figure 7.
MK2 is not upstream of Smad signaling in response to TGF-β. (A) TGF-β causes phosphorylation of both Smad2/3 and p38 in wild-type and MK2−/− MEF. Smad2/3 phosphorylation is observed before p38 phosphorylation. (B) Inhibiting the TGF-β Type I receptor kinase by SB431542 blocked Smad2/3 phosphorylation in both wild-type and MK2−/− MEF. SB431542 also blocked the activation of p38 by TGF-β.
DISCUSSION
Fibrosis occurs when fibroblasts and the extracellular matrix proteins they produce, such as collagen, accumulate in tissues, replacing normal tissue that is required for proper organ function. In pulmonary fibrosis, which can arise in response to drugs, asbestos, physical injury, inflammation, or unknown agents, the replacement of alveolar epithelium by fibrotic tissue results in inadequate gas exchange. Pulmonary fibrosis, particularly when due to unknown etiology (i.e., IPF), is usually progressive and fatal. Research on the role played by fibroblasts in fibrosis has increasingly focused on a differentiated form of fibroblasts, the myofibroblast. Because of their expression of smooth muscle–specific proteins, such as α-SMA, their known role in wound contraction and cytokine production, as well as their localization near fibrotic foci, myofibroblasts were suspected to promote fibrosis. Thus significant research has been conducted into the mechanism by which fibroblasts differentiate into myofibroblasts. In this article we describe an important role for the kinase MK2 in fibroblast differentiation into myofibroblasts in culture and in mice with pulmonary fibrosis. Furthermore, our results suggest that MK2 and myofibroblast formation might play a role in protection against rather than in causing fibrosis.
Myofibroblasts are believed to be derived from fibroblasts or epithelial cells through differentiation into a muscle cell–like phenotype, which is identified by expression of smooth muscle–specific proteins such as α-SMA. The classical profibrotic cytokine TGF-β, which is elevated in IPF lungs (5), causes fibroblasts to differentiate into myofibroblasts (10–13). As TGF-β has been central to pulmonary fibrosis pathogenesis (5, 29, 30), investigating its effect on fibroblasts was of particular interest. TGF-β belongs to a large family of proteins, including members such as bone morphogenetic proteins, that are implicated in a variety of diseases, such as cancer and pulmonary hypertension. It exerts its actions primarily through cell surface receptors that activate Smad proteins (for review, see Ref. 15). TGF-β has also been reported to activate Ras and Erk (16, 17), Rho GTPase and JNK (18), and p38 MAP kinase (19). Myofibroblast differentiation in response to TGF-β has been suggested to be mediated by p38, and inhibition of p38 has been reported to reduce pulmonary (20, 21) and renal (22) fibrosis in animal models. Yet some researchers reported no effect of inhibiting p38 on differentiation of lung fibroblasts into myofibroblasts (31).
p38 is activated by stimuli such as ultraviolet radiation and hyperosmolarity and a variety of cytokines and growth factors that lead to its phosphorylation. Activated p38 phosphorylates a variety of substrates, including the kinase MK2, leading to its activation. MK2 in turn phosphorylates additional substrates, such as the small heat shock protein HSP27. The latter event leads to actin stress fiber formation because phosphorylated HSP27 no longer blocks the polymerization of actin (32). We have investigated the p38-MK2-HSP27 pathway in endothelial cells and demonstrated that it mediates actin stress fiber formation in response to hypoxia (24, 33). Our laboratory has also recently demonstrated that fibroblasts that lack MK2 contain less actin stress fibers than wild-type fibroblasts (4). These fibroblasts also contained significantly less α-SMA than their wild-type counterparts. Formation of actin stress fibers, which is expected to be reduced in MK2−/− cells that cannot phosphorylate HSP27, has been associated with increased cell contractility, which in turn can induce α-SMA expression (34, 35). This effect, however, is not likely to explain the reduced α-SMA expression in MK2−/− MEF, because overexpressing phospho-mimicking HSP27 in these cells did not significantly increase α-SMA expression (data not shown).
Our recent manuscript also showed that the MK2−/− fibroblasts did not differ from wild-type cells in the activation of serum response element, which is one of the main pathways for α-SMA induction. However, MK2 disruption significantly reduced α-SMA mRNA stability (4), which is consistent with the known action of MK2 on cytokine expression (28, 36, 37). As we have performed these experiments in MEF, and since populations of fibroblasts are known to be heterogeneous in the level of α-SMA being expressed, we further tested the relation of α-SMA expression to MK2 by transfecting wild-type MEF with MK2 siRNA. The latter approach also caused a reduction in α-SMA expression (Figure 3A). On the other hand, when we overexpressed constitutively active MK2 in MK2−/− fibroblasts, α-SMA expression increased significantly. These results coupled with the observations made in mouse lungs argue against the observed difference in α-SMA level being due to variation in isolated MEF populations, or due to developmental effects on α-SMA expression in MK2−/− mice from which the MEF were isolated. Indeed, the effect of MK2 appears to be specific to fibroblasts in the sense that we have seen intense α-SMA staining in smooth muscle cells in lung airways and vasculature of MK2−/− mice.
As myofibroblasts are believed to play an important role in wound repair and fibrosis, we evaluated whether the MK2−/− mice differed in their susceptibility to pulmonary fibrosis induced by bleomycin treatment. The MK2−/− mice developed more severe fibrosis in response to belomycin than wild-type mice, as judged by the amount of collagen deposited in their lungs, as well as by immunohistochemical evidence including increased cellularity and Trichrome staining. While the accumulated cells in fibrotic foci of MK2−/− mice were stained positively for vimentin, a fibroblast marker, they were not significantly stained for α-SMA. On the other hand, fibrotic foci of wild-type mice contained cells that stained positively for both vimentin and α-SMA. Our findings suggest that MK2 is needed for myofibroblast differentiation in fibrotic injury, yet its absence and lack of myofibroblasts formation appear to exacerbate fibrosis instead of ameliorating it. While this conclusion is contrary to commonly expressed views on the role of myofibroblasts in pulmonary fibrosis, the evidence for myofibroblast involvement in pathogenesis has been more circumstantial than direct. For instance, while myofibroblasts have been consistently observed around the site of fibrotic injury, earlier reports noted that they are there in the early phase of injury and disappear as fibrosis develops (30). Thus myofibroblasts might be involved in the response to injury and play a protective role against fibrosis. Indeed, a very recent study of renal fibrosis in α-SMA–null mice seems to indicate that that is the case (38). Nevertheless, other myofibroblast markers and properties need to be evaluated in the MK2−/− mice and fibroblasts before definitive statements can be made concerning the importance of myofibroblasts in the development of fibrosis.
In attempting to understand the cellular properties that render MK2−/− mice more susceptible to fibrosis, we compared the proliferative properties of MK2−/− and wild-type MEF. As shown in Figure 5A, the MK2−/− cells do proliferate faster than wild-type cells, which might explain their excessive accumulation in fibrotic foci. MK2 disruption might cause higher proliferative rates through various mechanisms, for example, by inhibiting HSP27 phosphorylation, which has been linked with growth arrest of fibroblasts (39). In addition, the p38-MK2 pathway has been implicated in inhibiting proliferation through negative feedback of Ras signaling (40), and inhibiting p38 has been associated with hematopoeitic colony formation (41). It is noteworthy that kidney fibroblasts from α-SMA knockout mice also proliferate faster than wild-type kidney fibroblasts (38), indicating that reduction of α-SMA expression in MK2−/− fibroblasts might be causally related to their faster proliferation. Thus the higher proliferative rate of MK2−/− MEF, coupled with increased collagen production, might explain the higher fibrotic propensity observed in lungs of MK2−/− mice.
Another property that was different in the MK2−/− MEF is that their migration was slower than their wild-type counterparts. As revealed by the wounding assay (Figure 6), MK2−/− MEF were slower than wild-type MEF in repopulating a scratched part of a culture plate. Such behavior has been reported earlier in MK2−/− cells (28, 42). Since MK2 is known to alter cytoskeleton dynamics via modulating HSP27 phosphorylation, we tested whether the latter might explain the different migration properties. However, overexpressing phospho-mimicking HSP27 in MK2−/− MEF did not alter the migration significantly. Recently, MK2 has been shown to promote cell migration through its phosphorylation and activation of LIM kinase-1 (43). It is possible that lack of α-SMA in MK2−/− fibroblasts renders them less contractile and therefore less motile. However, other studies related α-SMA expression to reduced motility (44, 45), and a recent study on α-SMA knockout cells reported them to be more motile than wild-type cells (38). Thus, MK2 might alter migratory properties of MEF through multiple mechanisms, and we speculate that the defective migration in addition to higher proliferation rate of MK2−/− fibroblasts perturbs their wound closure abilities, and exacerbates their accumulation, leading to fibrosis.
At the molecular level, MK2 disruption does not appear to affect Smad signaling, but p38 activation and likely MK2 activation in response to TGF-β occur somewhere downstream of Smad2/3 (Figure 7). Thus MK2, which would be downstream of both kinases, might be one target that is perturbed in pulmonary and other types of fibrosis. Our results suggest that MK2 is necessary for proper fibroblast function and differentiation into myofibroblasts. Restoring that activity or increasing it through different approaches might be of therapeutic value in wound healing and fibrosis.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Howard Surks (Tufts-New England Medical Center), and Dr. Kazuo Maruyama (Tokyo Medical and Dental University) for providing the pMEpuro vector.
This work was supported by NIH Grant HL-79320 (to U.S.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0077OC on June 28, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society/European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Cherniack RM, Crystal RG, Kalica AR. NHLBI workshop summary. Current concepts in idiopathic pulmonary fibrosis: a road map for the future. Am Rev Respir Dis 1991;143:680–683. [DOI] [PubMed] [Google Scholar]
- 3.Vyalov SL, Gabbiani G, Kapanci Y. Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol 1993;143:1754–1765. [PMC free article] [PubMed] [Google Scholar]
- 4.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, Toksoz D, Kayyali US. Smooth muscle alpha-actin expression and myofibroblast differentiation by tgfbeta are dependent upon mk2. J Cell Biochem 2007;100:1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil N, O'Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 1991;5:155–162. [DOI] [PubMed] [Google Scholar]
- 6.Pan LH, Ohtani H, Yamauchi K, Nagura H. Co-expression of tnf alpha and il-1 beta in human acute pulmonary fibrotic diseases: an immunohistochemical analysis. Pathol Int 1996;46:91–99. [DOI] [PubMed] [Google Scholar]
- 7.Piguet PF, Ribaux C, Karpuz V, Grau GE, Kapanci Y. Expression and localization of tumor necrosis factor-alpha and its mrna in idiopathic pulmonary fibrosis. Am J Pathol 1993;143:651–655. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor {beta}1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta 1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 1993;68:696–707. [PubMed] [Google Scholar]
- 12.Roy SG, Nozaki Y, Phan SH. Regulation of [alpha]-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol 2001;33:723. [DOI] [PubMed] [Google Scholar]
- 13.Yokozeki M, Moriyama K, Shimokawa H, Kuroda T. Transforming growth factor-beta 1 modulates myofibroblastic phenotype of rat palatal fibroblasts in vitro. Exp Cell Res 1997;231:328–336. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 2003;14:538–546. [DOI] [PubMed] [Google Scholar]
- 15.Attisano L, Wrana JL. Signal transduction by the tgf-beta superfamily. Science 2002;296:1646–1647. [DOI] [PubMed] [Google Scholar]
- 16.Hartsough MT, Frey RS, Zipfel PA, Buard A, Cook SJ, McCormick F, Mulder KM. Altered transforming growth factor signaling in epithelial cells when ras activation is blocked. J Biol Chem 1996;271:22368–22375. [DOI] [PubMed] [Google Scholar]
- 17.Hartsough MT, Mulder KM. Transforming growth factor beta activation of p44[image] in proliferating cultures of epithelial cells. J Biol Chem 1995;270:7117–7124. [DOI] [PubMed] [Google Scholar]
- 18.Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of rho-like gtpases and stress-activated protein kinase/c-jun n-terminal kinase (sapk/jnk) in transforming growth factor beta -mediated signaling. J Biol Chem 1997;272:1429–1432. [DOI] [PubMed] [Google Scholar]
- 19.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J-i, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta -induced gene expression. J Biol Chem 1999;274:27161–27167. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka H, Arai T, Mori M, Goya S, Kida H, Morishita H, Fujiwara H, Tachibana I, Osaki T, Hayashi S. A p38 mapk inhibitor, fr-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2002;283:L103–L112. [DOI] [PubMed] [Google Scholar]
- 21.Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, Romanic AM, Adams JL, Hay DW, Griswold DE. Sb 239063, a p38 mapk inhibitor, reduces neutrophilia, inflammatory cytokines, mmp-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L895–L902. [DOI] [PubMed] [Google Scholar]
- 22.Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol 2004;15:370–379. [DOI] [PubMed] [Google Scholar]
- 23.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. Mapkap kinase 2 is essential for lps-induced tnf-alpha biosynthesis. Nat Cell Biol 1999;1:94–97. [DOI] [PubMed] [Google Scholar]
- 24.Kayyali US, Pennella CM, Trujillo C, Villa O, Gaestel M, Hassoun PM. Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on the map kinase-associated protein kinase mk2. J Biol Chem 2002;28:28. [DOI] [PubMed] [Google Scholar]
- 25.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, et al. Regulation of hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem 1999;274:18947–18956. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 28.Kotlyarov A, Gaestel M. Is mk2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression? Biochem Soc Trans 2002;30:959–963. [DOI] [PubMed] [Google Scholar]
- 29.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor {beta}1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991;88:6642–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapanci Y, Desmouliere A, Pache JC, Redard M, Gabbiani G. Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis: possible role of transforming growth factor beta and tumor necrosis factor alpha. Am J Respir Crit Care Med 1995;152:2163–2169. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. Transforming growth factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-jun-nh2-terminal kinase-dependent pathway. Am J Respir Crit Care Med 2001;163:152–157. [DOI] [PubMed] [Google Scholar]
- 32.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein hsp25 abolish its actin polymerization-inhibiting activity. J Biol Chem 1994;269:20780–20784. [PubMed] [Google Scholar]
- 33.An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 mapk- and rho kinase-dependent pathways. Am J Physiol Cell Physiol 2005;289:C521–C530. [DOI] [PubMed] [Google Scholar]
- 34.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 2001;159:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arora PD, Narani N, McCulloch CAG. The compliance of collagen gels regulates transforming growth factor-β induction of {alpha}-smooth muscle actin in fibroblasts. Am J Pathol 1999;154:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk H-D, Holtmann H, Kollias G, Gaestel M. Mk2 targets au-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem 2002;277:3065–3068. [DOI] [PubMed] [Google Scholar]
- 37.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mrna stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol 2006;26:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeji M, Moriyama T, Oseto S, Kawada N, Hori M, Imai E, Miwa T. Smooth muscle {alpha}-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J Biol Chem 2006;281:40193–40200. [DOI] [PubMed] [Google Scholar]
- 39.Arata S, Hamaguchi S, Nose K. Inhibition of colony formation of nih 3t3 cells by the expression of the small molecular weight heat shock protein hsp27: Involvement of its phosphorylation and aggregation at the c-terminal region. J Cell Physiol 1997;170:19–26. [DOI] [PubMed] [Google Scholar]
- 40.Chen G, Hitomi M, Han J, Stacey DW. The p38 pathway provides negative feedback for ras proliferative signaling. J Biol Chem 2000;275:38973–38980. [DOI] [PubMed] [Google Scholar]
- 41.Navas TA, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, Parmar S, Haghnazari E, Zhou L, Collins R, et al. Inhibition of overactivated p38 mapk can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood 2006;108:4170–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannigan MO, Zhan L, Ai Y, Kotlyarov A, Gaestel M, Huang CK. Abnormal migration phenotype of mitogen-activated protein kinase-activated protein kinase 2−/− neutrophils in zigmond chambers containing formyl-methionyl-leucyl-phenylalanine gradients. J Immunol 2001;167:3953–3961. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. Mapkapk-2-mediated lim-kinase activation is critical for vegf-induced actin remodeling and cell migration. EMBO J 2006;25:713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: Retardation of motility in fibroblasts. J Cell Biol 1996;134:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto-Inoue M, Kamada S, Kimura G, Taniguchi S. The induction of smooth muscle alpha actin in a transformed rat cell line suppresses malignant properties in vitro and in vivo. Cancer Lett 1999;142:173–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.