Abstract
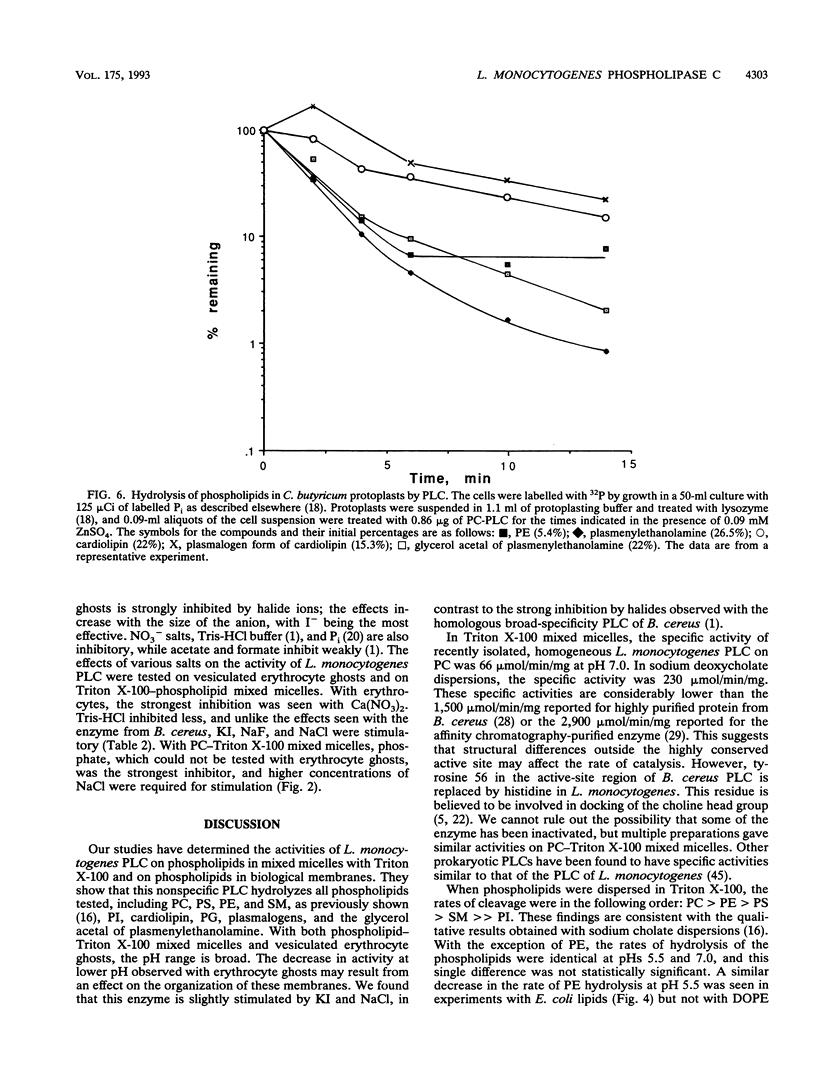
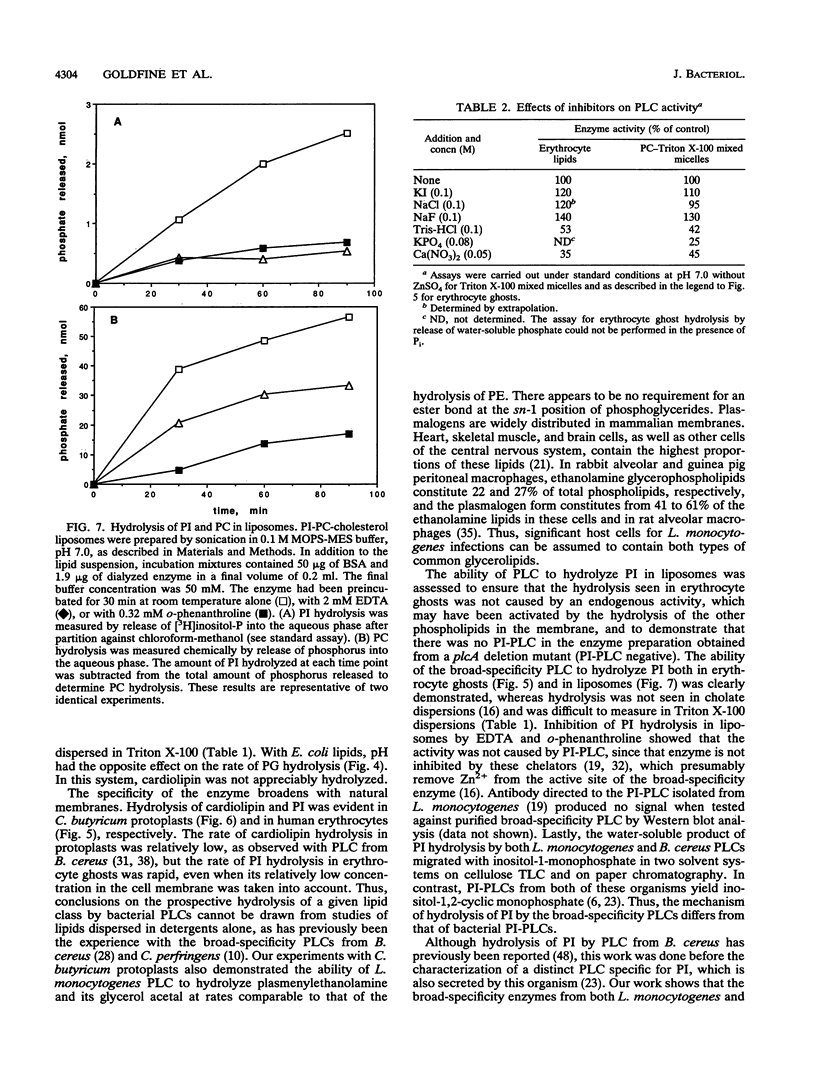
Listeria monocytogenes secretes a phospholipase C (PLC) which has 39% amino acid sequence identity with the broad-specificity PLC from Bacillus cereus. Recent work indicates that the L. monocytogenes enzyme plays a role during infections of mammalian cells (J.-A. Vazquez-Boland, C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart, Infect. Immun. 60:219-230, 1992). The homogeneous enzyme has a specific activity of 230 mumol/min/mg when phosphatidylcholine (PC) is dispersed in sodium deoxycholate. With phospholipid-Triton X-100 mixed micelles, the enzyme had a broad pH optimum between 5.5 and 8.0, and the rates of lipid hydrolysis were in the following order: PC > phosphatidylethanolamine (PE) > phosphatidylserine > sphingomyelin >> phosphatidylinositol (PI). Activity on PC was stimulated 35% by 0.5 M NaCl and 60% by 0.05 mM ZnSO4. When Escherichia coli phospholipids were dispersed in Triton X-100, PE and phosphatidylglycerol, but not cardiolipin, were hydrolyzed. The enzyme was active on all phospholipids of vesiculated human erythrocytes including PI, which was rapidly hydrolyzed at pH 7.0. PI was also hydrolyzed in PI-PC-cholesterol liposomes by the nonspecific PLC from L. monocytogenes and by the homologous enzyme from B. cereus. The water-soluble hydrolysis product was identified as inositol-1-phosphate. For the hydrolysis of human erythrocyte ghost phospholipids, a broad pH optimum was also observed. 32P-labelled Clostridium butyricum protoplasts, which are rich in ether lipids, were treated with PLC. The enzyme hydrolyzed the plasmalogen form of PE, its glycerol acetal, and cardiolipin, in addition to PE. I-, Cl- and F- stimulated activity on either PC- Triton X-100 mixed micelles or human erythrocyte ghosts, unlike the enzyme from B. cereus which is strongly inhibited by halides. Tris-HCl, phosphate, and calcium nitrate had similar inhibitory effects on the enzyme on the enzymes from L. monocytogenes and B. cereus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
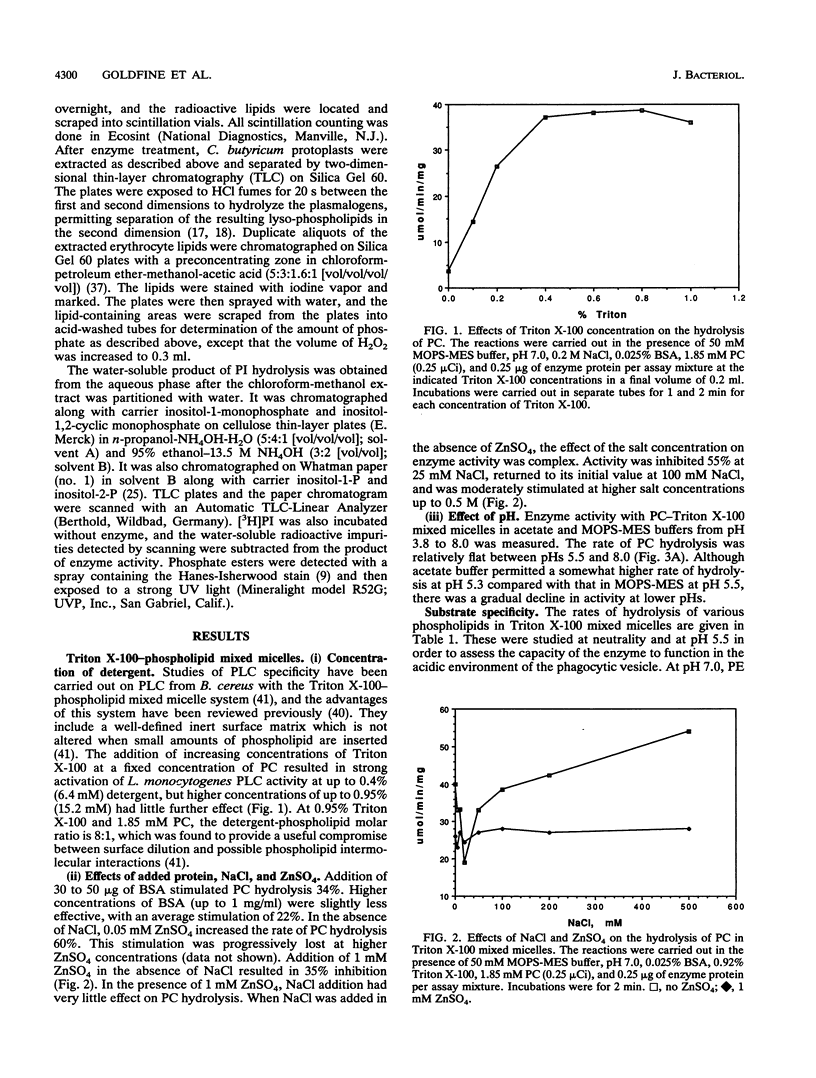
- Aakre S. E., Little C. Inhibition of Bacillus cereus phospholipase C by univalent anions. Biochem J. 1982 Jun 1;203(3):799–801. doi: 10.1042/bj2030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
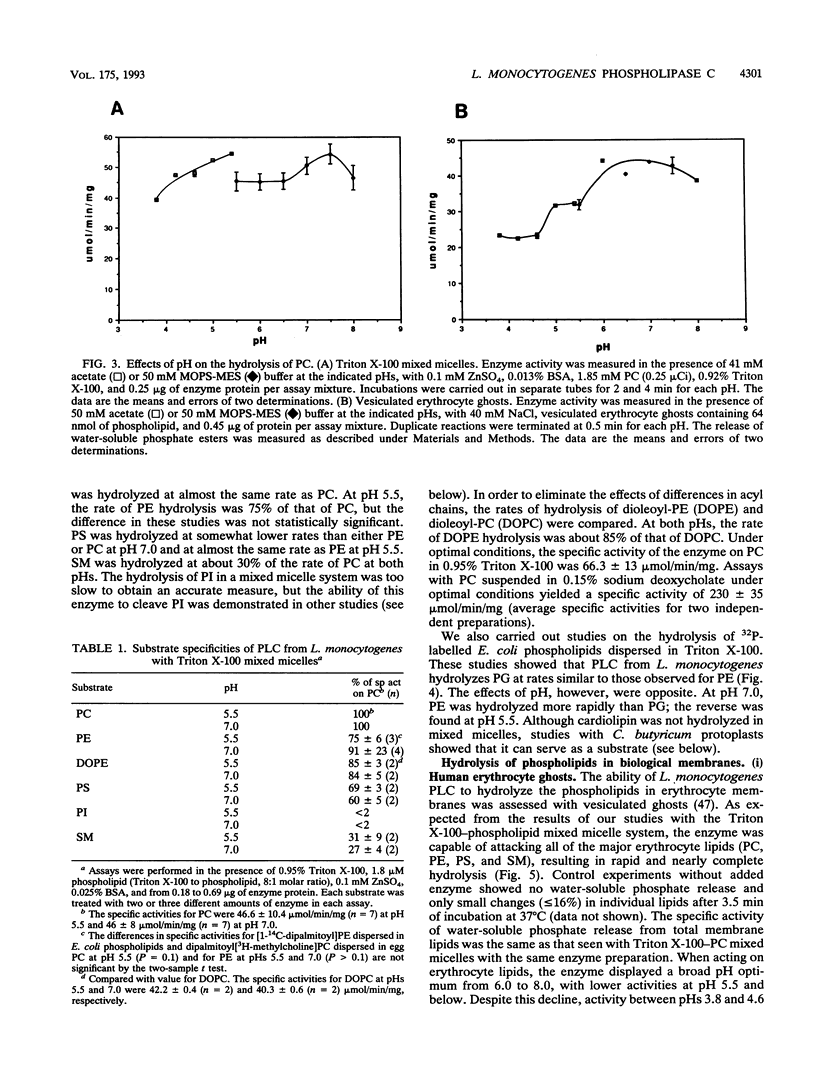
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byberg J. R., Jørgensen F. S., Hansen S., Hough E. Substrate-enzyme interactions and catalytic mechanism in phospholipase C: a molecular modeling study using the GRID program. Proteins. 1992 Apr;12(4):331–338. doi: 10.1002/prot.340120405. [DOI] [PubMed] [Google Scholar]
- Camilli A., Goldfine H., Portnoy D. A. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med. 1991 Mar 1;173(3):751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Tilney L. G., Portnoy D. A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993 Apr;8(1):143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag N. E., Youngman P., Portnoy D. A. Transcriptional activation of the Listeria monocytogenes hemolysin gene in Bacillus subtilis. J Bacteriol. 1992 Feb;174(4):1293–1298. doi: 10.1128/jb.174.4.1293-1298.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Frehel C., Gouin E., Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991 Jun 28;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Raveneau J., Beretti J. L., Lecroisey A., Vazquez-Boland J. A., Alouf J. E., Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991 Jul;59(7):2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
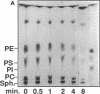
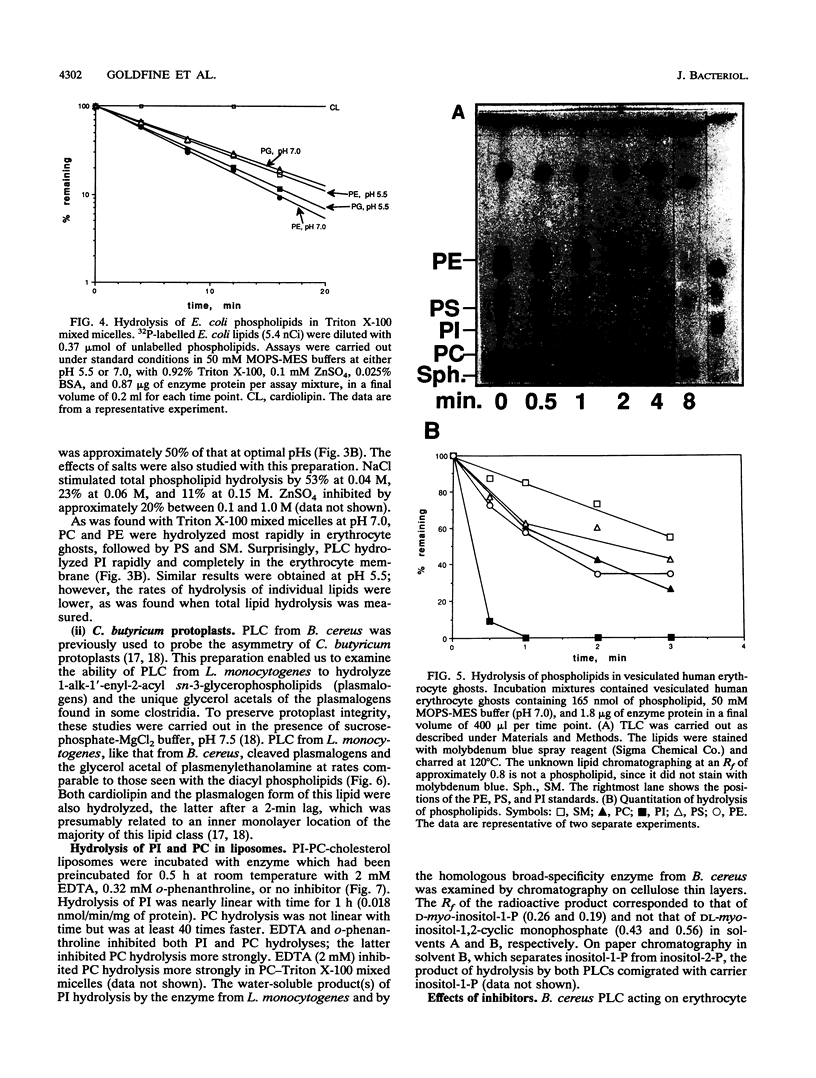
- Goldfine H., Johnston N. C., Bishop D. G. Ether phospholipid asymmetry in Clostridium butyricum. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1502–1507. doi: 10.1016/s0006-291x(82)80077-6. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Knob C. Purification and characterization of Listeria monocytogenes phosphatidylinositol-specific phospholipase C. Infect Immun. 1992 Oct;60(10):4059–4067. doi: 10.1128/iai.60.10.4059-4067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S., Hansen L. K., Hough E. Crystal structures of phosphate, iodide and iodate-inhibited phospholipase C from Bacillus cereus and structural investigations of the binding of reaction products and a substrate analogue. J Mol Biol. 1992 May 20;225(2):543–549. doi: 10.1016/0022-2836(92)90938-g. [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. Phospholipid aliphatic chain composition modulates lipid class composition, but not lipid asymmetry in Clostridium butyricum. Biochim Biophys Acta. 1985 Feb 28;813(1):10–18. doi: 10.1016/0005-2736(85)90339-6. [DOI] [PubMed] [Google Scholar]
- Kondo T. Preparation of microcapsules from human erythrocytes: use in transport experiments of glutathione and its S-conjugate. Methods Enzymol. 1989;171:217–225. doi: 10.1016/s0076-6879(89)71013-2. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Siess W. Measurement of inositol phospholipid turnover in platelets. Methods Enzymol. 1987;141:176–192. doi: 10.1016/0076-6879(87)41066-5. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991 Feb;5(2):361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Haffner C., Domann E., Goebel W., Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of listeria monocytogenes. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C., Aurebekk B., Otnaess A. B. Purification by affinity chromatography of phospholipase C from Bacillus cereus. FEBS Lett. 1975 Apr 1;52(2):175–179. doi: 10.1016/0014-5793(75)80800-3. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Bell R. M., Vagelos P. R. Effect of phospholipase C hydrolysis of membrane phospholipids on membranous enzymes. J Biol Chem. 1972 May 10;247(9):2835–2841. [PubMed] [Google Scholar]
- Mengaud J., Braun-Breton C., Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991 Feb;5(2):367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Dramsi S., Gouin E., Vazquez-Boland J. A., Milon G., Cossart P. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol Microbiol. 1991 Sep;5(9):2273–2283. doi: 10.1111/j.1365-2958.1991.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Waku K. The metabolism of glycerophospholipid and its regulation in monocytes and macrophages. Prog Lipid Res. 1989;28(3):205–243. doi: 10.1016/0163-7827(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Ostroff R. M., Wretlind B., Vasil M. L. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun. 1989 May;57(5):1369–1373. doi: 10.1128/iai.57.5.1369-1373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas A. A., Mullins R. E., Gadsden R. H. Improved one-dimensional thin-layer chromatography of phospholipids in amniotic fluid. Clin Chem. 1982 Jan;28(1):209–211. [PubMed] [Google Scholar]
- Paton J. C., May B. K., Elliott W. H. Membrane phospholipid asymmetry in Bacillus amyloliquefaciens. J Bacteriol. 1978 Aug;135(2):393–401. doi: 10.1128/jb.135.2.393-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L. J., Washburn W. N., Deems R. A., Dennis E. A. Assay strategies and methods for phospholipases. Methods Enzymol. 1991;197:3–23. doi: 10.1016/0076-6879(91)97129-m. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Otnaess A. B., Kensil C. A., Dennis E. A. The specificity of phospholipase A2 and phospholipase C in a mixed micellar system. J Biol Chem. 1978 Feb 25;253(4):1252–1257. [PubMed] [Google Scholar]
- Shortridge V. D., Lazdunski A., Vasil M. L. Osmoprotectants and phosphate regulate expression of phospholipase C in Pseudomonas aeruginosa. Mol Microbiol. 1992 Apr;6(7):863–871. doi: 10.1111/j.1365-2958.1992.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland J. A., Kocks C., Dramsi S., Ohayon H., Geoffroy C., Mengaud J., Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992 Jan;60(1):219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B. Phospholipase C (phosphatidylcholine cholinephosphohydrolase, EC 3.1.4.3) from Bacillus cereus. Methods Enzymol. 1974;32:154–161. doi: 10.1016/0076-6879(74)32019-8. [DOI] [PubMed] [Google Scholar]