Abstract
Brefeldin A (BFA) treatment stops secretion and leads to the resorption of much of the Golgi apparatus into the endoplasmic reticulum. This effect is reversible upon washing out the drug, providing a situation for studying Golgi biogenesis. In this investigation Golgi regeneration in synchronized tobacco BY-2 cells was followed by electron microscopy and by the immunofluorescence detection of ARF1, which localizes to the rims of Golgi cisternae and serves as an indicator of COPI vesiculation. Beginning as clusters of vesicles that are COPI positive, mini-Golgi stacks first become recognizable 60 min after BFA washout. They continue to increase in terms of numbers and length of cisternae for a further 90 min before overshooting the size of control Golgi stacks. As a result, increasing numbers of dividing Golgi stacks were observed 120 min after BFA washout. BFA-regeneration experiments performed on cells treated with BFA (10 μg mL−1) for only short periods (30–45 min) showed that the formation of ER-Golgi hybrid structures, once initiated by BFA treatment, is an irreversible process, the further incorporation of Golgi membranes into the ER continuing during a subsequent drug washout. Application of the protein kinase A inhibitor H-89, which effectively blocks the reassembly of the Golgi apparatus in mammalian cells, also prevented stack regeneration in BY-2 cells, but only at very high, almost toxic concentrations (>200 μm). Our data suggest that under normal conditions mitosis-related Golgi stack duplication may likely occur via cisternal growth followed by fission.
As with other membranous organelles, the Golgi apparatus is inherited in similar numbers and size in daughter cells after mitosis. Since the Golgi apparatus is dependent upon the continual delivery and recycling of membrane and lumenal cargo from the endoplasmic reticulum (ER), one way for the Golgi apparatus to keep pace with nuclear and cell division would be via additional de novo formation through the initial homotypic fusion of ER-derived vesicles (Glick, 2002; Bonifacino and Glick, 2004; LaPointe et al., 2004). A good example for this is the yeast Pichia pastoris, in which new Golgi stacks are formed immediately adjacent to new ER export sites in young buds (Bevis et al., 2002). Another possibility that can be envisaged is that of fission, occurring either perpendicular or parallel to the major cisternal axis, with cisternal growth or multiplication taking place prior or subsequent to the division of the Golgi apparatus (Warren, 1993). This scenario has been well demonstrated in a number of parasitic flagellates that possess only a single Golgi stack (Warren, 1993; Shorter and Warren, 2002; He et al., 2004; for references, see Pelletier et al., 2002).
According to Shorter and Warren (2002), the presence of a cell wall is a decisive factor in determining how a Golgi apparatus is duplicated and partitioned in the offspring. In plants and yeast secretion continues throughout the life cycle, and indeed in the case of plants may even increase in activity during cell plate formation (Nebenführ et al., 2000). As a consequence, the Golgi apparatus in plants is continually present during mitosis. In contrast, secretion is not required until very late in cytokinesis in animal cells, so that here Golgi inheritance follows the general pattern of dispersal of the individual Golgi stacks constituting the perinuclear Golgi complex before and during prophase, followed by fragmentation of the stacks to produce a “haze” of small vesicles that then partition between the daughter cells and reassemble to new Golgi stacks in late telophase (Shorter and Warren, 2002; Altan-Bonnet et al., 2004; Barr, 2004). Much controversy has surrounded the role of the ER in this process, in particular, whether the Golgi haze consists of ER-distinct Golgi remnants or whether it represents aggregates of Golgi proteins within the ER (Zaal et al., 1999; Axelsson and Warren, 2004; Pecot and Malhotra, 2004). However, recently published work on both yeast (Reinke et al., 2004) and mammalian cells (Altan-Bonnet et al., 2006) has firmly established that the postmitotic reconstruction of the Golgi apparatus is dependent upon the reactivation of ER export activity.
Although the GTPases Sar1 and Arf1 locate to the ER and the Golgi apparatus, respectively, the activities of both are essential for the maintenance of structure and function of the Golgi apparatus in animal as well as plant cells. This is apparent from experiments in which the expression of dominant-negative mutants of both GTPases leads to the disassembly/fragmentation of the Golgi apparatus (Miles et al., 2001; Ward et al., 2001). Thus, the existence of the Golgi apparatus is dependent upon the correct functioning of vesiculating activities both at the ER (COPII via Sar1) and at the Golgi apparatus (COPI via Arf1). In accordance with this, Golgi fragmentation at the onset of mitosis in animal cells is initiated by a sequential inactivation of Arf1 and Sar1 activities (Altan-Bonnet et al., 2003, 2006). As such, the breakdown and reassembly of the Golgi apparatus during mitosis in animal cells closely resemble the situation in interphase cells when brefeldin A (BFA) is added and then washed out, since this drug is well known to prevent the activation of Arf1 (Jackson and Casanova, 2000), and leads both in plants and animals to a redistribution of a significant portion of the Golgi apparatus into the ER (Klausner et al., 1992; Nebenführ et al., 2002). This provides the legitimation for several recent studies on Golgi biogenesis in mammalian cells, where de novo regeneration has been followed in cells recovering from treatment with BFA and H-89, a protein kinase inhibitor that blocks Sar1 recruitment and therefore prevents COPII vesicle formation (Aridor and Balch, 2000; Puri and Linstedt, 2003; Bejarano et al., 2006).
Depending upon the relative expression of BFA-sensitive to -insensitive Arf-GEFs (GTP exchange factors) and their subcellular location, the primary target of BFA action in some plant cells appears to lie on the endocytic pathway rather than the Golgi apparatus (Geldner, 2004; Richter et al., 2007). However, for tobacco BY-2 cells, this is not the case. This model plant cell reacts rapidly to low concentrations of BFA, initially forming distinctive hybrid structures from ER membranes and Golgi cisternae and subsequently resulting in the complete disappearance of the Golgi apparatus (Ritzenthaler et al., 2002; Saint-Jore et al., 2002; Robinson and Ritzenthaler, 2006). As monitored by the visualization of mannosidase I-GFP (a cis-located glycoprotein-processing enzyme) and sialyltransferase (ST)-GFP (a trans-Golgi marker), the Golgi apparatus recovers fully within 2 to 3 h of washing out the BFA (Ritzenthaler et al., 2002; Saint-Jore et al., 2002), but a detailed investigation into the mechanism of de novo Golgi stack regeneration in BY-2 cells has not been performed.
Using synchronized wild-type BY-2 cells, we have followed the course of Golgi stack formation and growth during regeneration from BFA by conventional and immunogold electron microscopy with plant-specific COPI and COPII antisera. In addition, we have monitored by confocal microscopy the growth of regenerating Golgi stacks in wild-type BY-2 cells by immunofluorescence labeling with ARF1 antibodies and in a transgenic cell line expressing the Golgi marker ST-GFP (Saint-Jore et al., 2002). Our data indicate that mini-Golgi stacks arise out of COPI-positive tubular-vesicle clusters, and then enlarge both in terms of cisternal number and length. In wild-type cells the regenerating Golgi stacks continue to grow over a 2- to 2.5-h period, reaching a size almost double that of normal stacks, before dividing. In the transgenic cell line, this process took over an hour longer. Golgi stack regeneration does not require cytoskeleton elements, but is inhibited by H-89, albeit at very high concentrations.
RESULTS
ARF1 Immunolabeling of BY-2 Golgi Stacks Regenerating after BFA Washout Points to Sequential Phases of Growth and Division
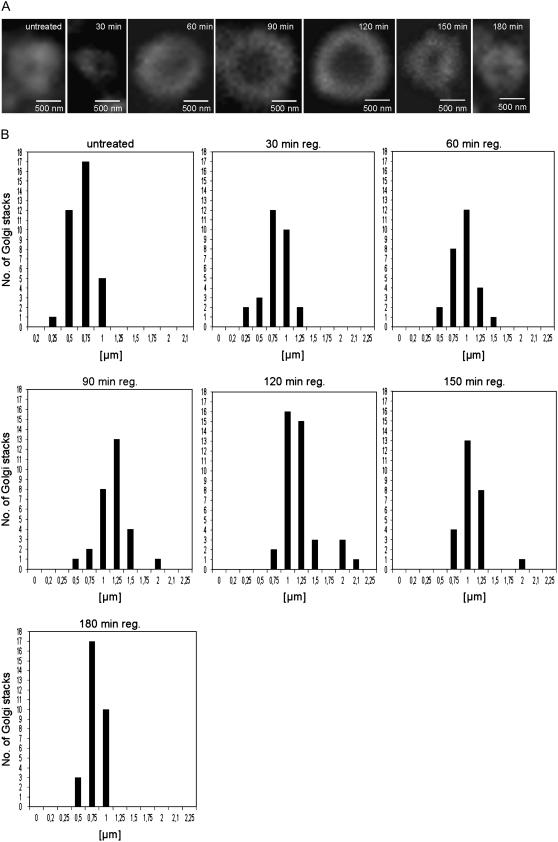
In preliminary time-course experiments where samples from synchronized BY-2 cultures were processed for conventional electron microscopy, we determined that a 2-h treatment with BFA (10 μg mL−1) was required to eliminate completely all traces of Golgi membranes (data not shown, but see similar results reported in Ritzenthaler et al., 2002). Unlike Arabidopsis (Arabidopsis thaliana) root cells where ARF1 localizes to both endosomes and the Golgi apparatus (Xu and Scheres, 2005), in tobacco BY-2 cells ARF1 is restricted to the rims of Golgi cisternae, where COPI vesicles are formed (Pimpl et al., 2000; Ritzenthaler et al., 2002; Yang et al., 2005; Robinson and Ritzenthaler, 2006). We have therefore used ARF1 immunofluorescence as a way of monitoring the reconstitution of BY-2 Golgi stacks during BFA washout. A clear ARF1 signal with a punctate appearance was obtained 30 min after the onset of regeneration (Fig. 1). After 30 min BFA washout, the doughnut-type signal typical for control Golgi stacks viewed 90° to the main cisternal axis gradually became recognizable with the inner unstained center continuing to enlarge up until regeneration periods of about 2 h. At this time the doughnut signal was almost twice the size (1.25 μm, comparing external doughnut diameters) of that measured for Golgi stacks in control cells (0.65 μm; Fig. 1). However, by 150 min regeneration the average doughnut size began to decrease and by 3 h of regeneration had attained a diameter corresponding to Golgi stacks in control cells (Fig. 1).
Figure 1.
Golgi stack regeneration during BFA washout in BY-2 cells as monitored by immunofluorescence labeling with ARF1 antibodies. A, Representative individual images from the recovery time points indicated. B, The external diameters of stacks visualized end-on were determined from at least 50 stacks from different cells in samples removed from cultures at the recovery times indicated.
Because the Arf1 immunofluorescence measurements were performed manually, the sample numbers per time point were not large. We therefore performed the same kind of BFA-regeneration experiment on a synchronized BY-2 cell line expressing the trans-Golgi marker ST-GFP (Saint-Jore et al., 2002). In this case, Golgi size was monitored digitally with the help of ImageJ (Supplemental Fig. S1). This time, the average Golgi diameter continued to increase (by 40% compared to the control cells) up to 3 h after BFA washout, before dropping to a value below that of the control cells. Interestingly, a comparison of the ARF1 immunofluorescence and ST-GFP signals indicated that the size of the Golgi stacks in the transgenic cell line was over double that of wild-type cells. This may be a consequence of the transgenic situation; indeed, the average diameter of Golgi cisternae in electron micrographs of nonsynchronized ST-GFP BY-2 cells lies around 0.9 μm (E. Hummel, unpublished data). However, it might in part be due to the location of the fluorescent signal being measured, the trans-cisternae with the ST-GFP signal perhaps having a larger diameter than the cis-cisternae and medial cisternae where the majority of ARF1 signal can be expected to be located. Nevertheless, in both cell types these observations strongly suggest that the cisternae in Golgi stacks regenerating de novo after BFA washout overshoot in terms of cisternal length those of untreated cells, before decreasing to normal size.
Pre-Golgi Vesicle Clusters and Mini-Golgis Are Characteristic Stages in Golgi Apparatus Regeneration
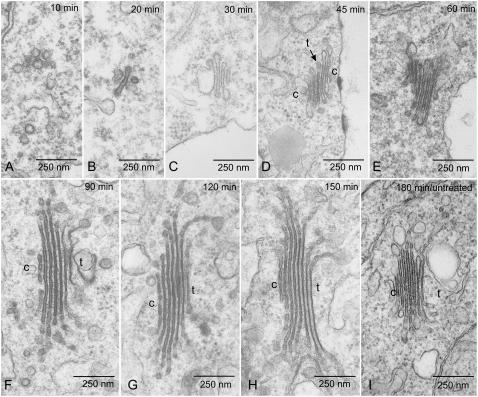
Observations made on thin sections of chemically fixed wild-type BY-2 cells collected at intervals during a 3-h regeneration from BFA treatment confirm the immunofluorescence results reported above. The first indication of Golgi reformation was witnessed by small clusters of 100-nm vesicles in cells after 10 min BFA washout (Fig. 2A). We were not able to determine a special spatial relationship of these clusters to the ER. Nevertheless, in samples processed by high-pressure freezing/freeze substitution, individual budding profiles on ER cisternae of the type previously reported by us in BY-2 cells (see figure 5, C and D, in Ritzenthaler et al., 2002) were observed (see Supplemental Fig. S2). These profiles were nevertheless very rare, making an immunogold determination of their possible COPII character virtually impossible (see below).
Figure 2.
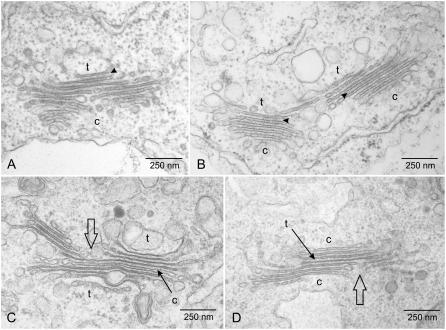
Electron microscopy of Golgi stack regeneration. A to I, Representative images of pre-Golgi structures (A and B), mini-Golgis (C–E), and mega-Golgis (F–H) in comparison to a Golgi stack from a wild-type cell not treated with BFA (I). The times at which samples were removed for chemical fixation are given as indicated. Cisternal indicators of polarity were easily recognizable after 90 min of BFA washout but could be occasionally discerned earlier. The Golgi stack presented in D, for instance, is an early example of a cis-trans-cis bipolar stack (see also Fig. 3D). c = cis; t = trans.
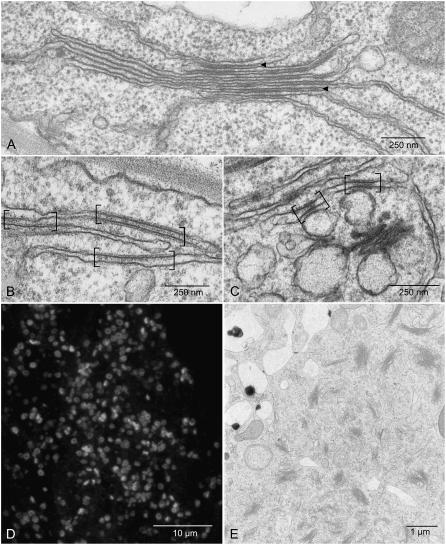
Figure 5.
Golgi regeneration after short-term BFA treatment. A, Stacked ER-Golgi hybrid present in wild-type BY-2 cells after 30 min of treatment with 10 μg mL−1 BFA. Intercisternal filaments are visible at different positions throughout the stack. B, Golgi domains in the ER (in brackets) remain recognizable 30 min after BFA washout. C, New Golgi stacks arise although old Golgi domains in the ER are still visible (30 min BFA → 45 min washout). D and E, Massive Golgi stack regeneration after short-term BFA treatment (30 min BFA → 120 min washout; D, confocal image after ARF1 immunostaining; E, chemical-fixed sample.
After 20 min BFA washout, individual, small cisternae began to appear that terminated in coated buds (Fig. 2B). With increasing washout times, mini-Golgi stacks appeared with initially three to four cisternae of approximately 200 nm length (Fig. 2C). The number of cisternae in the mini-Golgi stacks increased to six to seven by 1 h BFA washout (Fig. 2, D and E), after which time the numbers remained constant. Parameters of stack polarity, e.g. cisternal lumen width and presence of intercisternal filaments between trans-most cisternae (Robinson and Kristen, 1982), also first became clearly visible after 1 h of BFA washout (Fig. 2, compare E with F). However, the cisternae continued to elongate (Fig. 2, F–H), reaching a maximum length of around 0.8 μm after 120 to 150 min (Fig. 2H; Table I). In contrast, the majority of Golgi stacks in 180 min washout samples had a size comparable to those in control cells (0.4 μm; Fig. 2I; Table I).
Table I.
Changing Golgi stack parameters during recovery from BFA treatment
| Regeneration Time | No. of Cisternae per Golgi Stacka | Diameter of Centermost Cisternab |
|---|---|---|
| min | nm | |
| 30 | 4 ± 1 | 200 ± 24 |
| 60 | 7 ± 1 | 290 ± 32 |
| 90 | 7 ± 2 | 630 ± 19 |
| 120 | 7 ± 1 | 760 ± 42 |
| 150 | 8 ± 1 | 400 ± 36 |
The sample size for these measurements ranged between 20 and 30 Golgi stacks.
These diameters are smaller than those measured by ARF1 fluorescence (see Fig. 1) and reflect the central, continuous portion of the cisterna rather than the vesiculating rim.
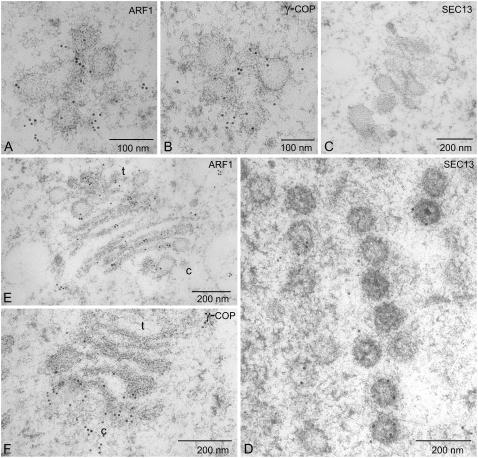
Sections from high-pressure frozen/freeze-substituted samples were processed for immunogold electron microscopy with Arabidopsis COPI (ARF1, γ-COP) and COPII (Sec13) antisera. The pre-Golgi tubular-vesicular clusters labeled positively with ARF1 and γ-COP antibodies (Fig. 3, A and B). In contrast, no labeling was obtained with the Sec13 antiserum (Fig. 3C). However, the SEC13 antibodies did label nuclear pores (Fig. 3D), confirming our previous demonstration that, as in yeast cells, SEC13 is a component of the nuclear pore complex Nup84 (Yang et al., 2005). This observation provides a good internal control for the lack of COPII on the pre-Golgi structures. Golgi stacks after 60 min regeneration (mini-Golgis) label with both ARF1 and γ-COP antisera, but distinctively (Fig. 3, E and F). Whereas γ-COP was restricted to the cis-pole of the stacks, ARF1 was detected at both the cis- and trans-poles, presumably corresponding to the role of ARF1 in the recruitment of clathrin-associated AP-1 adaptors at the trans-Golgi network as well as in coatomer recruitment at the cis-Golgi (Owen et al., 2004).
Figure 3.
Immunogold electron microscopy with COPI and COPII antisera. Sections of BY-2 cells prepared by high-pressure freezing/freeze substitution and stained with ARF1, γ-COP for the presence of COPI proteins, and SEC13 for COPII proteins. Samples were removed and freeze-fixed after 20 min (A–D) and 60 min (E and F). c = cis; t = trans.
Electron Microscopy of Regenerating BY-2 Golgi Confirms Fission of Stacks
Numerous examples of dividing Golgi stacks were found in samples fixed after 2 h BFA washout. Interestingly, in addition to cis → trans fission profiles (Fig. 4, A and B), which could be expected on the basis of partial inhibition of homotypic fusion of ER-derived pre-Golgi elements (Rossanese and Glick, 2002), we observed dividing Golgi stacks with an anomalous bipolar architecture. In some cases, the outermost cisternae had a typical trans morphology with small cisternal lumina and the innermost cisterna was of the cis-type (Fig. 4C). In other cases, the outermost and innermost cisternae had cis and trans morphologies, respectively (Fig. 4D).
Figure 4.
Fission profiles of dividing Golgi stacks found in BY-2 cells 120 to 150 min after BFA washout. In addition to putative cis → trans profiles (A and B), divisions in bipolar stacks were also observed (C and D). c = cis; t = trans.
De Novo Golgi Stack Regeneration Occurs Even in the Presence of BFA-Induced ER-Golgi Hybrids
As previously documented, BFA treatment leads temporarily to the formation of ER-Golgi hybrid stacks in BY-2 cells (Ritzenthaler et al., 2002; Robinson and Ritzenthaler, 2006). These structures are visible after 20 to 45 min of BFA (10 μg mL−1) treatment and can be recognized by the intercisternal filaments lying between the stacked ER/Golgi cisternae (Fig. 5A). When, after a short pulse of BFA (30–45 min), the drug is washed out, the stacks disassemble but the sites where the Golgi cisternae have been assimilated into the ER continue to be visible for at least a further 30 min (Fig. 5, B and C). These Golgi “domains” are characterized by (1) an intercisternal filament-like coat on both sides of the ER membrane, (2) an electron-opaque lumenal content, and (3) a “stiffness” of the membranes compared to the bordering ER. Interestingly, these domains are still recognizable during the early stages of de novo Golgi regeneration (Fig. 5C). These observations indicate that, once Golgi and ER membranes have begun to fuse as a result of BFA treatment, the incorporation of the Golgi membranes into the body of the ER continues and is irreversible even after drug washout.
Also arising out of these BFA washout experiments was the observation that recovery from short-term BFA treatments (30–45 min) led to considerably higher Golgi stack densities than after the longer exposures to BFA (120 min), which were necessary to abolish completely all traces of the Golgi apparatus (compare Fig. 5, D and E, with figure 1 in Ritzenthaler et al., 2002; figure 8, A and D, in Yang et al., 2005; see also Table II). This might simply reflect the physiological status of the cells but constitutes a potentially exploitable effect for future studies on the de novo formation of the plant Golgi apparatus.
Table II.
Relative Golgi stack densities in 45-nm optical sections taken through the cell cortex of tobacco BY-2 cells
Golgi stacks were identified by ARF1 immunofluorescence.
| Treatment | Golgi Stack Density |
|---|---|
| 45 min BFA-treated cells | Approximately 1.7 Golgi/10 μm2 |
| 120 min BFA-treated cells | Approximately 1.2 Golgi/10 μm2 |
| Untreated cells | Approximately 1.1 Golgi/10 μm2 |
Effects of Inhibitors on Golgi Regeneration
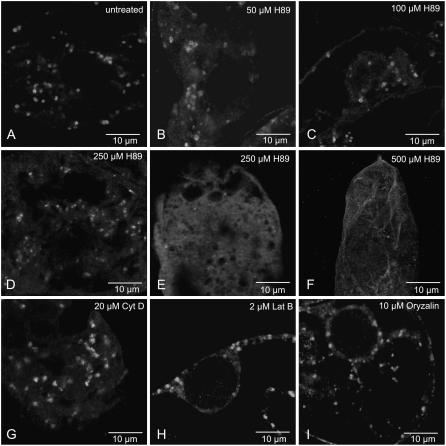
We tested the effects of two different classes of inhibitors known to interfere with Golgi structure in mammalian cells on Golgi regeneration after BFA treatment: the protein kinase inhibitor H-89 (Lee and Linstedt, 2000; Bejarano et al., 2006) and the cytoskeleton inhibitors oryzalin (for microtubules), cytochalasin D, and latrunculin B (for actin microfilaments; Laporte et al., 2003). BY-2 cells were monitored for Golgi recovery by ARF1 immunofluorescence after 120 min BFA washout in the presence or absence (control) of the inhibitors. At concentrations known to effectively depolymerize the respective elements (see Supplemental Fig. S3; see also Laporte et al., 2003), the cytoskeleton inhibitors were without effect on stack regeneration (Fig. 6, G–I). H-89, at the maximal concentration usually applied to mammalian cells (50 μm), was also without effect. However, when presented at 250 μm, a considerable reduction (50%) in the percentage of cells showing Golgi stacks (Fig. 6D) was observed. Correspondingly, the percentage of cells with an increased diffuse cytosolic fluorescence (Fig. 6E) increased by 50% (Table III). In those cells showing Golgi-like fluorescence, the punctae were smaller and the typical doughnut image was no longer visible (Fig. 6, compare D with A and G). Concentrations higher than 350 μm invariably led to a shrinkage of the cytoplasm (Fig. 6F). Vitality tests confirmed that concentrations of H-89 above 250 μm were extremely toxic to BY-2 cells, but also demonstrated that even at the highest concentrations used the solvent dimethyl sulfoxide (DMSO) had no toxic effects (Supplemental Fig. S4).
Figure 6.
Effects of inhibitors on Golgi stack regeneration. BY-2 cells were treated with BFA (10 μg mL−1) for 45 min, then allowed to regenerate Golgi stacks in the presence or absence of the indicated inhibitor for a 120-min period. Golgi stack presence was monitored by ARF1 immunofluorescence. Images presented are from 45-μm optical sections in the confocal laser scanning microscope. A, Control cells. B to F, Increasing concentrations of H-89. G and H, Microfilament inhibitors cytochalasin D (20 μm) and latrunculin B (2 μm). I, Microtubule inhibitor oryzalin (10 μm).
Table III.
Relative presence of Golgi stacks in tobacco BY-2 cells recovering from BFA treatment (10 μg mL−1) in the presence/absence of inhibitors
Golgi stacks were monitored by ARF1 immunofluorescence, whereby cells were nominally classified as having punctate fluorescence (representing Golgi-bound ARF1) or a diffuse fluorescence (representing cytosolic ARF1). Sample sizes were usually 100 to 200 cells.
| Treatment | Percentage of Cells with Labeled Golgi Stacks | Percentage of Cells with Labeled Cytosol |
|---|---|---|
| Control | 65 ± 5 | 35 ± 5 |
| 50 μm H-89 | 60 ± 5 | 40 ± 5 |
| 100 μm H-89 | 55 ± 5 | 45 ± 5 |
| 250 μm H-89 | 40 ± 5 | 60 ± 5 |
| 500 μm H-89 | 15 ± 5 | 85 ± 5 |
| 20 μm cytochalasin D | 60 ± 5 | 40 ± 5 |
DISCUSSION
ER Vesiculation and Pre-Golgi Structures
The morphological manifestation of ER exit sites and their relationship to plant Golgi stacks is a controversial issue (compare daSilva et al., 2004; Yang et al., 2005; Hanton et al., 2007; Hummel et al., 2007; for reviews, see also Hanton et al., 2005; Aniento et al., 2006; Robinson et al., 2007). Also in question is whether anterograde ER-Golgi transport in plants is accomplished by COPII vesicles or by direct tubular connections (Hawes, 2005; Hawes and Satiat-Jeunemaitre, 2005). The results presented here are pertinent to these ongoing discussions. In our studies we have never observed anything resembling a tubular outgrowth between the ER and the vesicle clusters that represent the first pre-Golgi elements in BY-2 cells recovering from BFA. On the other hand, we have detected budding profiles at the surface and at the terminal ends of the ER (Supplemental Fig. S2). However, unlike the alga Chlamydomonas where COPII vesiculation is concentrated at special domains of the ER (transitional ER; Hummel et al., 2007), these budding profiles were singular structures and were relatively rarely observed, which precluded their immunogold identification as COPII. If in fact they do represent the nascent COPII vesicles required for the formation of pre-Golgi elements, it is strange that they were recorded so infrequently.
Our immunogold labeling studies on the pre-Golgi vesicle clusters are consistent with the notion that ERGIC in mammalian cells is formed from the homotypic fusion of ER-derived COPII vesicles and functions in the COPI-based retrieval of escaped ER resident proteins (Aridor et al., 1995; Scales et al., 1997; Horstmann et al., 2002; Murshid and Presley, 2004). Thus, in contrast to COPI-coat proteins, COPII-coat proteins were detected only at very low levels in these vesicle clusters, since the latter would have been discarded prior to fusion. The presence of COPI-coat proteins presumably reflects the inherent facility of Golgi membranes to bud COPI vesicles as soon as they are formed.
Golgi Regeneration Is Not Cytoskeleton Dependent
Since the Golgi apparatus in mammalian cells is usually located perinuclear and the ER is arranged in the cell cortex (Lippincott-Schwartz et al., 2000), transport in the early secretory pathway has a long-range component that is absent in plant cells (Hanton et al., 2005; Aniento et al., 2006). ER-to-Golgi transport of proteins in mammalian cells is facilitated by an intermediate compartment, ERGIC, which morphologically resembles a cluster of vesicles and tubules (Horstmann et al., 2002). Although there is some controversy over whether the ERGIC itself or membrane carriers derived from it traverse between the ER to the Golgi complex (compare Ben-Tayaka et al., 2004, with Murshid and Presley, 2004), it is clear that this transport occurs along microtubules. In fact, the actual process of ER exit is tightly coupled to the presence of microtubules via an interaction between the COPII-coat protein Sec23 and dynein/dynactin motor protein complexes (Watson et al., 2004). In distinct contrast, ER-to-Golgi transport in plants occurs across a short interface and is independent of microtubules (Nebenführ et al., 2000; Saint-Jore et al., 2002). Instead, plant Golgi stacks travel along actin filaments that are situated parallel to the surface of the ER (for references, see Hawes, 2005).
On the basis of FRAP experiments, newly synthesized Golgi enzymes seem to be successfully transported from the ER to the Golgi irrespective of whether the actin cytoskeleton is intact, and Golgi stacks are moving (Brandizzi and Hawes, 2004), or depolymerized, and the Golgi stacks are immobile (Brandizzi et al., 2002). However, on the basis of data presented here and elsewhere (Saint-Jore et al., 2002), the regeneration of a new Golgi stack out of the ER is also independent of the actin cytoskeleton. How then do ER-derived pre-Golgi elements nucleate to eventually form mini-Golgis as we have described above? A reasonable speculation is the existence of a BFA-resistant template, as described for mammalian cells (Ward et al., 2001; Barr and Short, 2003). Matrix proteins, or Golgins, which make up such a template, have been found in plant cells (Latijnhouwers et al., 2005), but the lack of availability of antibodies against these proteins has prevented us from testing this hypothesis.
High Concentrations of Protein Kinase A Inhibitors Are Required to Prevent Golgi Regeneration in Plants
Protein kinase A (PKA) is a tetrameric enzyme that associates with the surface of Golgi membranes in mammalian cells (Nigg et al., 1985). PKA has been shown to be important for the formation of transport vesicles at the trans-Golgi network (Muniz et al., 1997) and is also required for Golgi reassembly after mitosis (Bejarano et al., 2006). It has been thought that PKA activity is required for ER export, based on the inhibition of Sar1 recruitment by the isoquinoline sulfonamide H-89, a Ser/Thr kinase inhibitor (Aridor and Balch, 2000). However, more recent data suggest that kinases other than PKA are involved in COPII recruitment to the ER (Palmer et al., 2005; Bejarano et al., 2006). Nevertheless, sequential treatments with BFA and H-89 have become a standard procedure to eliminate both the Golgi apparatus and ER exit sites in mammalian cells (Puri and Linstedt, 2003; Bejarano et al., 2006). H-89 at the highest concentrations normally applied to mammalian cells (50 μm) had no effect on Golgi regeneration in BY-2 cells. Only when present at 250 μm was an inhibition observed (Fig. 6, D and E). Concentrations higher than 300 μm proved toxic. Interestingly, and unlike the situation in mammalian cells, 250 μm H-89 did not cause a change in Golgi morphology when monitored in a BY-2 cell line expressing the fluorescent Golgi marker GONST1-YFP (data not shown).
Golgi Fission during BFA Regeneration: Consequences
Data on Golgi inheritance in higher plant cells are sparse and contradictory. According to Garcia-Herdugo et al. (1988), Golgi stacks double in number during metaphase in onion (Allium cepa) root meristems. In contrast, Golgi duplication was claimed to occur during cytokinesis in synchronized cultures of Catharanthus roseus (Hirose and Komamine, 1989). However, working with BY-2 cells, Nebenführ et al. (2000) were unable to allocate a distinctive increase in Golgi stack numbers to any particular phase in the cell cycle. Nevertheless, in a more recent paper in which stereology and electron tomography were applied to shoot meristem cells of Arabidopsis, Segui-Simarro and Staehelin (2006) pinpointed exactly the cell cycle stage in which Golgi duplication occurred: G2, immediately prior to the onset of mitotic prophase. This finding is in agreement with previous publications dealing with green algae (Chida and Noguchi, 1989; Ueda, 1997). Enlargement followed by division before the onset of mitosis is also characteristic of the Golgi apparatus in the apicomplexan parasite Toxoplasma gondii (Pelletier et al., 2002).
It is often assumed that Golgi multiplication in higher plants occurs by the division of preexisting stacks because clear data in support of de novo formation are lacking. Although the de novo regeneration of the Golgi apparatus during recovery from BFA treatment is obviously an artificial situation, it does indicate that this process might be used to increase Golgi stack numbers prior to the onset of mitosis. Despite this possibility, our analyses of the regeneration of Golgi stacks in BY-2 cells (here) and in Chlamydomonas (Hummel et al., 2007) nevertheless indicate that plant Golgi stacks also have a propensity for lateral enlargement of their cisternae, followed by vertical division of the stack. As a result there are now several scenarios by which the plant Golgi apparatus can duplicate in number before mitosis begins. The two extremes are: (1) solely through de novo biogenesis from the ER, and (2) exclusively by growth and fission of pre-existing Golgi stacks. However, various combinations of both mechanisms are quite possible. If Golgi multiplication involves cisternal growth followed by division, this would demand that membrane trafficking to and from the Golgi apparatus must be modified in such a way to allow the cisternae to elongate. This could be achieved by a temporary reduction in secretory and retrograde COPI vesicle production, during which time anterograde traffic out of the ER remains unaffected. Monitoring for the activities of the coat protein-recruiting GTPases ARF1 and SAR1 throughout the cell cycle might be a means of testing this hypothesis.
MATERIALS AND METHODS
Synchronizing Cell Cultures, BFA Treatment, and Washout
Wild-type tobacco BY-2 (Nicotiana tabacum var. Bright Yellow 2) cells were cultivated by shaking (120 rpm) in the dark in Murashige and Skoog's medium (Murashige and Skoog, 1962) at 27°C. The suspension-cultured cells were maintained in the log-phase subculturing weekly into fresh medium at a dilution of 1:50. The procedure of Samuels et al. (1998) was used to synchronize cultures: 20-mL cell suspension from a 7-d-old culture was incubated with aphidicoline (final concentration 3 μg mL−1) for 24 h, then washed with a 4% (w/v) Suc solution and resuspended in 50 mL of fresh growth medium. After 6 h the presence of metaphase cells was checked by staining with DAPI (Sigma) according to Otto (1994), before addition of propyzamide (final concentration 3.5 μm). The cells were incubated for 16 h at 18°C, washed with 4% Suc, and then incubated for a further 3 h in fresh medium. At this stage 90% to 95% of the cells had just entered interphase. BFA (final concentration 10 μg mL−1) was then added and the cells incubated at 25°C for the times given. For regeneration studies, aliquots of cells were removed from the treatment medium and washed free of BFA with 4% Suc before incubation in fresh growth medium.
Vitality Test
The possible toxic effects of H-89 and the solvent DMSO were tested on BY-2 cells using the fluorescein diacetate (FDA) procedure of Widholm (1972). Different concentrations of these substances were given to log-phase cultures and incubated for 60 min at 25°C. Aliquots of the cell suspension were then removed and stained with FDA (10 μm) before observation under a Zeiss Axiovert LSM510 using a ×10/0.3 Plan-Neoflaur objective and excitation/emission wavelengths of 488/528 to 539 nm. Vitality was monitored in terms of percentage of living cells.
Immunofluorescence Labeling with ARF1 Antibodies and Confocal Microscopy
Samples of cells regenerating from BFA were removed from the washout medium at the times indicated and fixed with 1.5% glutaraldehyde in culture medium for 15 min at room temperature. Further processing was performed exactly as given previously by Ritzenthaler et al. (2002). For immunostaining, cells were first incubated with ARF1 antibodies (Pimpl et al., 2000) at a primary dilution of 1:1,000 in phosphate buffered saline containing 0.1% (v/v) acetylated bovine serum albumin (Aurion), and after washing then incubated with Alexa-Fluor 488 goat anti-rabbit IgG (Molecular Probes) diluted 1:100. For further experimental details and final preparations for confocal laser scanning microscopy, see Ritzenthaler et al. (2002). Cells were observed under a Zeiss Axiovert LSM510 microscope using a ×63 1.2 NA water immersion objective with optical sections of 45 μm thickness, and excitation/emission wavelengths of 488/505 to 530 nm.
Confocal Imaging and Measurements with Stable Transformed ST-GFP BY-2 Cells
ST-GFP BY-2 cultures (a line similar to Saint Jore et al., 2002) were synchronized following the protocol described by Samuels et al. (1998). Confocal imaging was done with LSM Zeiss Meta and 63× water objective lens. Before taking z-stacks saturation was adjusted with the range indicator in Zeiss software (to prevent oversaturation of Golgi signal). Pinhole was always set to 1. Measurements of Golgi stacks were carried out with ImageJ measurement plugins. In total more than a thousand Golgi were measured.
Conventional Electron Microscopy and Immunogold Labeling of High-Pressure Frozen/Freeze-Substituted Specimens with COPI and COPII Antisera
Glutaraldehyde-osmium-fixed samples were prepared exactly as given by Ritzenthaler et al. (2002), with thin sections of Spurr's resin-embedded specimens poststained in methanolic uranyl acetate and Reynold's lead acetate. Samples were high-pressure frozen using a Baltec HPF010 freezer and freeze-substituted in the absence of osmium using an AFS apparatus (Leica) as given by Tse et al. (2004). Thin sections from HM-20 (Polysciences)-embedded specimens were then processed for immunogold electron microscopy by standard procedures (Lam et al., 2007). For COPI labeling affinity-purified ARF1 and γ-COP antibodies (Movafeghi et al., 1999; Pimpl et al., 2000) and for COPII labeling SAR1 and SEC13 antibodies (Yang et al., 2005), at primary dilutions of 1:25, were used. Sections were observed in a Philips CM10 electron microscope operating at 80 kV.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Golgi apparatus regeneration monitored in BY-2 cells expressing ST-GFP.
Supplemental Figure S2. Individual budding profiles on the ER of BY-2 cells recovering (20 min) from BFA treatment. To enhance contrast, the sections were stained with a 1% KMnO4 solution for 1 min.
Supplemental Figure S3. Disruption of cytoskeletal elements in protoplasts from BY-2 cells.
Supplemental Figure S4. FDA vitality tests for DMSO and H-89 on BY-2 cells.
Supplementary Material
Acknowledgments
The technical assistance of Ms. Steffi Gold (Heidelberg) and Anne Kearns (Oxford) is greatly appreciated.
This work was supported by the German Research Council (DFG grant no. Ro440/11–3).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David G. Robinson (david.robinson@urz.uni-heidelberg.de).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Altan-Bonnet N, Phair RD, Polishchuk RS, Weigert R, Lippincott-Schwartz J (2003) A role for Arf1 in mitotic Golgi disassembly, chromosome segregation, and cytokinesis. Proc Natl Acad Sci USA 100 13314–13319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J (2004) Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol 16 364–372 [DOI] [PubMed] [Google Scholar]
- Altan-Bonnet N, Sougrat R, Liu W, Snapp EL, Ward T, Lippincott-Schwartz J (2006) Golgi inheritance in mammalian cells is mediated through endoplasmic reticulum export activities. Mol Biol Cell 17 990–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Matsuoka K, Robinson DG (2006) ER-to-Golgi transport: the COPII-pathway. In DG Robinson, ed, The Plant Endoplasmic Reticulum. Springer-Verlag, Berlin, pp 99–124
- Aridor M, Balch WE (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem 275 35673–35676 [DOI] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE (1995) Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol 131 875–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson MA, Warren G (2004) Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze. Mol Biol Cell 15 1843–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA (2004) Golgi inheritance: shaken but not stirred. J Cell Biol 164 955–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Short B (2003) Golgins in the structure and dynamics of the Golgi apparatus. Curr Opin Cell Biol 15 405–413 [DOI] [PubMed] [Google Scholar]
- Bejarano E, Cabrera M, Vega L, Hidalgo J, Velasco A (2006) Golgi structural stability and biogenesis depend on associated PKA activity. J Cell Sci 119 3764–3775 [DOI] [PubMed] [Google Scholar]
- Ben-Tayaka H, Miura K, Pepperkok R, Hauri HP (2004) Live cell imaging of bidirectional traffic from the ERGIC. J Cell Sci 118 357–367 [DOI] [PubMed] [Google Scholar]
- Bevis BJ, Hammond AT, Reinke CA, Glick BS (2002) De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol 4 750–756 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116 153–166 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Hawes C (2004) A long and winding road. EMBO Rep 5 245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C (2002) Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14 1293–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Noguchi T (1989) Multiplication of the dictyosome during the formation of autospores in the green alga Chlorococcum infusionum. Biol Cell 65 189–194 [PubMed] [Google Scholar]
- daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16 1753–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Herdugo G, Gonzalez-Reyes JA, Garcia-Navarro F, Navas P (1988) Growth kinetics of the Golgi apparatus during the cell cycle in onion root meristems. Planta 175 305–312 [DOI] [PubMed] [Google Scholar]
- Geldner N (2004) The plant endosomal system—its structure and role in signal transduction and plant development. Planta 219 547–560 [DOI] [PubMed] [Google Scholar]
- Glick BS (2002) Can the Golgi form de novo? Nat Rev Mol Cell Biol 3 615–619 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Bortolotti LE, Renna L, Stefano G, Brandizzi F (2005) Crossing the divide—transport between the endoplasmic reticulum and Golgi apparatus in plants. Traffic 6 267–277 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Renna L, Matheson LA, Brandizzi F (2007) De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiol 143 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes C (2005) The plant Golgi apparatus. New Phytol 165 29–44 [DOI] [PubMed] [Google Scholar]
- Hawes C, Satiat-Jeunemaitre B (2005) The plant Golgi apparatus—going with the flow. Biochim Biophys Acta 1744 93–107 [DOI] [PubMed] [Google Scholar]
- He CY, Ho HH, Malsam J, Chalouni C, West CM, Ullu E, Toomre D, Warren G (2004) Golgi duplication in Trypanosoma brucei. J Cell Biol 165 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S, Komamine A (1989) Changes in ultrastructure of Golgi apparatus during the cell cycle in a synchronous culture of Catharanthus roseus. New Phytol 111 599–605 [DOI] [PubMed] [Google Scholar]
- Horstmann H, Ng CP, Tang BL, Hong W (2002) Ultrastructural characterization of endoplasmic reticulum—Golgi transport containers (EGTC). J Cell Sci 115 4263–4273 [DOI] [PubMed] [Google Scholar]
- Hummel E, Schmickl R, Hinz G, Hillmer S, Robinson DG (2007) Brefeldin A action and recovery in Chlamydomonas are rapid and involve fusion and fission of Golgi cisternae. Plant Biol 9 489–501 [DOI] [PubMed] [Google Scholar]
- Jackson CL, Casanova JE (2000) Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol 10 60–67 [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L (2007) Rice SCAMP1 defines clathrin-coated, trans-golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19 296–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe P, Gurkan C, Balch WE (2004) Mise en place—this bud's for the Golgi. Mol Cell 14 413–414 [DOI] [PubMed] [Google Scholar]
- Laporte C, Vetter G, Loudes A-M, Robinson DG, Hillmer S, Stussi-Garaud C, Ritzenthaler C (2003) Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of grape vine fanleaf virus movement protein in tobacco BY-2 cells. Plant Cell 15 2058–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latijnhouwers M, Hawes C, Carvalho C (2005) Holding it all together? Candidate proteins for the plant Golgi matrix. Curr Opin Plant Biol 8 632–639 [DOI] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD (2000) Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol Biol Cell 11 2577–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol 16 557–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles S, McManus H, Forsten KE, Storrie B (2001) Evidence that the entire Golgi apparatus cycles in interphase HeLa cells: sensitivity of Golgi matrix proteins to an ER exit block. J Cell Biol 155 543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movafeghi A, Happel N, Pimpl P, Tai GH, Robinson DG (1999) Arabidopsis Sec21p and Sec23p homologs. Probable coat proteins of plant COP-coated vesicles. Plant Physiol 119 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz M, Martin ME, Hidalgo J, Velasco A (1997) Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci USA 94 14461–14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for growth and rapid bioassays with tobacco tissue culture. Physiol Plant 15 473–494 [Google Scholar]
- Murshid A, Presley JF (2004) ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell Mol Life Sci 61 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Frohlick JA, Staehelin LA (2000) Redistribution of Golgi stacks and other organelles during mitosis and cytokinesis in plant cells. Plant Physiol 124 135–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA, Hilz H, Eppenberger HM, Dutly F (1985) Rapid and reversible translocation of the catalytic subunit of cAMP-dependent protein kinase type II from the Golgi complex to the nucleus. EMBO J 4 2801–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto FJ (1994) High resolution analysis of nuclear DNA employing fluorochrome DAPI. Methods Cell Biol 41 211–217 [DOI] [PubMed] [Google Scholar]
- Owen DJ, Collins BM, Evans PR (2004) Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol 20 153–191 [DOI] [PubMed] [Google Scholar]
- Palmer KJ, Konkel JE, Stephens DJ (2005) PCTAIRE protein kinases interact directly with the COPII complex and modulate secretory cargo transport. J Cell Sci 118 3839–3847 [DOI] [PubMed] [Google Scholar]
- Pecot MY, Malhotra V (2004) Golgi membranes remain segregated from the endoplasmic reticulum during mitosis in mammalian cells. Cell 116 99–107 [DOI] [PubMed] [Google Scholar]
- Pelletier L, Stern CA, Pypaert M, Sheff D, Ngo HM, Roper N, He CY, Hu K, Toomre D, Coppens I, et al (2002) Golgi biogenesis in Toxoplasma gondii. Nature 418 548–552 [DOI] [PubMed] [Google Scholar]
- Pimpl P, Movafeghi A, Coughlan S, Denecke J, Hillmer S, Robinson DG (2000) In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12 2219–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Linstedt AD (2003) Capacity of the Golgi apparatus for biogenesis from the endoplasmic reticulum. Mol Biol Cell 14 5011–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke CA, Kozik P, Glick BS (2004) Golgi inheritance in small buds of Saccharomyces cerevisiae is linked to endoplasmic reticulum inheritance. Proc Natl Acad Sci USA 101 18018–18023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Geldner N, Schrader J, Wolters H, Stierhof YD, Rios G, Koncz C, Robinson DG, Juergens G (2007) Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature 448 488–492 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenfuhr A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C (2007) Membrane dynamics in the early secretory pathway. CRC Crit Rev Plant Sci 26 199–225 [Google Scholar]
- Robinson DG, Kristen U (1982) Membrane flow via the Golgi apparatus of higher plant cells. Int Rev Cytol 77 89–127 [Google Scholar]
- Robinson DG, Ritzenthaler C (2006) Imaging the early secretory pathway in BY-2 cells. In T Nagata, K Matsuoka, D Inzé, eds, Tobacco BY-2 Cells: From Cellular Dynamics to Omics, Vol 58. Biotechnology in Agriculture and Forestry. Springer-Verlag, Heidelberg, pp 135–151
- Rossanese OW, Glick BS (2002) Deconstructing Golgi inheritance. Traffic 2 589–596 [DOI] [PubMed] [Google Scholar]
- Saint-Jore CM, Evins J, Batoko H, Brandizzi F, Moore I, Hawes C (2002) Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J 29 661–678 [DOI] [PubMed] [Google Scholar]
- Samuels AL, Meehl J, Staehelin LA (1998) Optimizing conditions for tobacco BY2-cell cycle synchronization. Protoplasma 202 2232–2236 [Google Scholar]
- Scales SJ, Pepperkok R, Kreis T (1997) Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90 1137–1148 [DOI] [PubMed] [Google Scholar]
- Segui-Simarro JM, Staehelin LA (2006) Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: a quantitative and spatial analysis. Planta 223 223–236 [DOI] [PubMed] [Google Scholar]
- Shorter J, Warren G (2002) Golgi architecture and inheritance. Annu Rev Cell Dev Biol 18 379–420 [DOI] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K (1997) The synchronous division of dictyosomes at the premitotic stage. Ann Bot (Lond) 80 29–33 [Google Scholar]
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J (2001) Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol 155 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G (1993) Membrane partitioning during cell division. Annu Rev Biochem 62 323–348 [DOI] [PubMed] [Google Scholar]
- Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ (2004) Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol 7 48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JM (1972) The use of fluorescein diacetate and phenosafrine for determining viability of cultured plant cells. Stain Technol 47 189–194 [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-ribosylation factor 1 function in epidermal cell polarity. Plant Cell 17 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, el Amawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG (2005) Visualization of COPII and Golgi dynamics in Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with ER exit sites (ERES). Plant Cell 17 1513–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal KJ, Smith CL, Polishchuk RS, Altan N, Cole NB, Ellenberg J, Hirschberg K, Presley JF, Roberts TH, Siggia E, et al (1999) Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell 99 589–601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.