2,6-Dichlorobenzonitrile (DCB; Fig. 1A) was reported to inhibit cellulose synthesis more than 30 years ago (Hogetsu et al., 1974) and has subsequently been used in numerous studies (e.g. Montezinos and Delmer, 1980; Edelmann and Fry, 1992; Shedletzky et al., 1992; Suzuki et al., 1992; Vaughn et al., 1996; Nakagawa and Sakurai, 1998; Sabba et al., 1999; Anderson et al., 2002; Himmelspach et al., 2003; Ohmiya et al., 2003; Talbot et al., 2007). However, because the mode of action of the drug is unknown, interpretation of the effects of DCB on cellulose synthesis has been ambiguous because of the possibility of indirect effects. Therefore, we have used live-cell imaging of transgenic plants carrying a yellow fluorescent protein (YFP)-labeled cellulose synthase 6 (CESA6) protein to examine the short-term effects of DCB on cellulose synthesis. These experiments showed that DCB rapidly inhibited the motility of YFP∷CESA6 complexes at the cortical z-plane in Arabidopsis (Arabidopsis thaliana) cells and caused hyperaccumulation of CESA complexes at distinct sites at the cell cortex. By contrast, the drug isoxaben (Heim et al., 1990; Fig. 1B) has previously been reported to clear YFP∷CESA6 from the membrane (Paredez et al., 2006).
Figure 1.
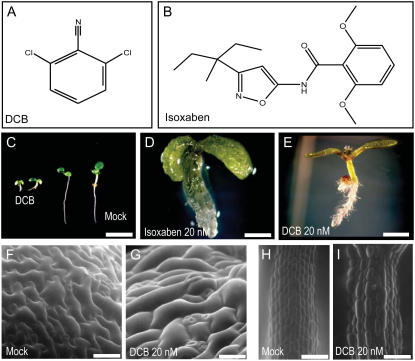
DCB and isoxaben treatment. A and B, Structure of DCB (A) and isoxaben (B). C, Dwarfed Arabidopsis seedling caused by treatment with 1 μm DCB compared with mock (0.1% dimethyl sulfoxide) after 5 d of growth. Scale bar = 2.5 mm. D, Plant growth on 20 nm isoxaben results in severely swollen organs on all parts of the plant. Scale bar = 500 μm. E, Same concentration of DCB readily induces swollen roots and hypocotyls. Scale bar = 2.5 mm. F to I, Environmental scanning electron microscopy images of cotyledon epidermal cells (F and G) and upper hypocotyl cells (H and I) of Arabidopsis seedlings observed after 3 d of growth. Cells treated with DCB (G and I) display altered morphogenesis and cell swelling compared with mock cells (F and H). Scale bars = 100 μm.
Consistent with previous reports of DCB and isoxaben action (Hogetsu et al., 1974; Scheible et al., 2001), Arabidopsis seedlings grown on drug-supplemented agar displayed a dwarfed seedling phenotype (Fig. 1C) characterized by severely swollen organ morphology in rapidly expanding tissues, such as those in hypocotyls and roots (Fig. 1, D and E). Environmental scanning electron microscopy of DCB-treated Arabidopsis cotyledon epidermal cells (Fig. 1, F and G) revealed loss of cell shape uniformity and severely swollen cells. In rapidly expanding upper hypocotyl cells, even at low DCB concentrations (20 nm), cells displayed severe bulging (Fig. 1, H and I) consistent with previous reports (Desprez et al., 2002). We also observed that plants carrying loss-of-function mutations in genes required for cell wall synthesis (CESA2, COBRA, KORRIGAN, and CESA6; summarized in Somerville, 2006) were hypersensitive to 20 nm DCB and 2 nm isoxaben (data not presented). The isoxaben-resistant Arabidopsis mutants carrying point mutations in the ixr1 and ixr2 genes (Heim et al., 1990) were indistinguishable from wild type in their ability to grow on agar supplemented with DCB at various concentrations (data not presented). Thus, DCB and isoxaben appear to act through different mechanisms.
UDP-Glc is the substrate for both callose synthase (Nishimura et al., 2003) and CESA (Somerville, 2006). Therefore, to evaluate the possibility that DCB inhibits cellulose synthesis by inhibiting UDP-Glc synthesis, we stained for callose (Brundrett et al., 1988) after treatment with DCB or isoxaben. The formation of callose punctae in the hypocotyl and cotyledons of whole Arabidopsis seedlings was observed after the addition of either drug (Supplemental Fig. S1), consistent with findings of Lukowitz et al. (2001) and Desprez et al. (2002). PMR4 encodes a callose synthase responsible for the production of callose in response to pathogens and wounding (Nishimura et al., 2003). Results presented here indicate that PMR4 is also responsible for isoxaben and DCB induced callose deposition (Supplemental Fig. S1).
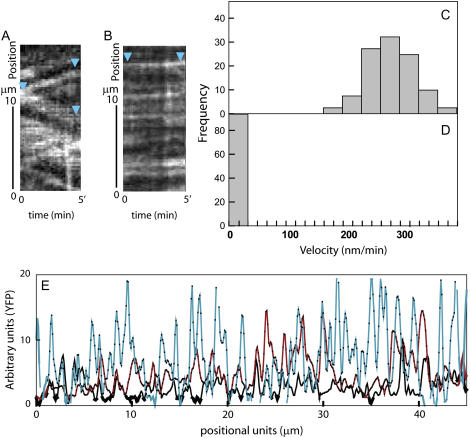
Kymograph analysis of time-lapse confocal images revealed that individual YFP∷CESA6 complexes traveled at 280 ± 32 nm min−1 through the plasma membrane in the mock-treated seedlings (Fig. 2, A and C), consistent with previous measurements taken on 2.5-d-old Arabidopsis seedlings in the upper hypocotyl region (DeBolt et al., 2007). Quantitative live-cell imaging of etiolated seedlings indicated that within 30 min following treatment with 5 μm DCB, YFP∷CESA6 complexes in the plasma membrane ceased movement (Fig. 2, B and D; Supplemental Movie S1). Maximal particle velocity following 30 min of treatment was 34 nm min−1, and the majority of labeled complexes at the plasma membrane focal plane displayed no measurable particle movement (Fig. 2D; Supplemental Movie S1). Cessation of CESA motility was observed during extended DCB exposure (up to 5 h).
Figure 2.
Quantitative analysis of YFP∷CESA6 shows that DCB causes cessation of CESA motility and hyperaccumulation of CESA at regions within the plasma membrane. A and B, Kymograph analysis of YFP∷CESA6 particles at the plasma membrane. CESA displacement over time is dramatically reduced under DCB (B) compared with mock (A). Scale bar = 2.5 μm. C and D, Histogram of particle velocities from 30 min of treatment with mock (C) or DCB (D). Mock-treated cells show a mean velocity of 280 nm/min, whereas particle velocity was nearly zero after DCB application. E, Cross-sectional intensities of YFP∷CESA6 emission. Cells were treated with mock for 2 h (black line), DCB for 30 min (red line), or DCB for 2 h (light blue line). During DCB treatment, YFP emission increased over time. Peaks indicate YFP∷CESA6 punctae at the plasma membrane.
In addition to inhibiting CESA particle velocity, DCB treatment also increased the intensity of YFP∷CESA6 punctae at the plasma membrane. To quantify this observation, we plotted YFP intensity values along linear transects on time-averaged image series. In these plots, sharp local maxima, or peaks, are representative of CESA particles at the cell cortex (Fig. 2E). In a time-course experiment, the mean peak height after 30 min of DCB treatment was 26% higher than that measured at 0 min (P < 0.001 Wilcoxon signed-rank test; Fig. 2E). After 150 min, the mean difference was up to 48% higher (Fig. 2E; based on n = 456 per treatment). Thus, DCB increases the accumulation of YFP∷CESA6 at discrete sites at the cell cortex.
To address the possibility that a portion of the immobile and particulate YFP∷CESA6 label accumulating at the cell cortex under DCB treatment might be label in vesicular compartments (Paredez et al., 2006) accumulating just under the plasma membrane, we followed YFP∷CESA6 complexes during the onset of DCB inhibition while maintaining the same cortical focal plane (Fig. 3, B and C). In these experiments, other labeled subcellular compartments such as the Golgi and an unknown compartment (Paredez et al., 2006) were clearly distinguishable as distinct from the stopped YFP∷CESA6 at the plasma membrane focal plane (Supplemental Movie S1). These observations extend previous observations by Herth (1987) who reported, on the basis of freeze-fracture electron micrographs, that addition of DCB to wheat (Triticum aestivum) roots stimulated accumulation of more than double the number of CESA rosettes in the plasma membrane. If the wheat root and Arabidopsis hypocotyls share drug target commonality, these results imply that YFP∷CESA label accumulates as rosettes in the plasma membrane upon DCB treatment.
Figure 3.
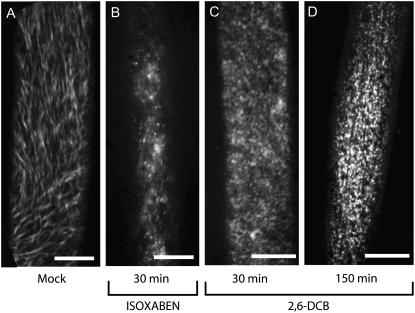
Live-cell imaging of YFP∷CESA6 in cells treated with mock (A), isoxaben (B), or DCB (C and D). Images represent time-averaged projections of 61 frames spaced 5 s apart. Movement of mock-treated YFP∷CESA6 gives rise to strands of YFP emission (A), whereas isoxaben clears the YFP∷CESA6 label from the plasma membrane focal plane after 30 min (B). In contrast, DCB causes CESA to accumulate at the plasma membrane after 30 min of treatment (C). Complexes are nonmotile and, therefore, are seen as punctae in the time-averaged image (C). After 150 min of DCB treatment (D), YFP∷CESA6 punctae appear brighter at the plasma membrane when compared with C. Scale bars = 10 μm.
It has been previously reported that the exogenous addition of sitosterol-β-glucoside (SSG) has the capacity to reverse the inhibition properties of 25 μm DCB in isolated cotton (Gossypium hirsutum) fibers (Peng et al., 2002). We attempted to repeat this result using the YFP∷CESA6 protein fusion and live-cell imaging as an assay for cellulose synthesis. Under the conditions reported by Peng et al. (2002) and over a broader range of drug concentrations (0.5–25 μm DCB and 7–21 μm SSG), no difference in particle velocity was detected between DCB alone and DCB + SSG (Wilcoxon signed-rank test [n = 90]; Supplemental Fig. S2). Since we have no evidence for SSG uptake, the results might be due to reduced SSG uptake by Arabidopsis hypocotyl cells compared to cotton fibers.
The pattern of mock-treated YFP∷CESA6 distribution and mobility at the plasma membrane (Fig. 3A) was rapidly (<30 min) disturbed upon treatment with isoxaben (Fig. 3B) and DCB (Fig. 3, C and D), compatible with the possibility that DCB may act directly on cellulose synthesis. Hyperaccumulation of CESA at discrete sites caused by DCB treatment can be clearly visualized as brighter punctae when comparing 150-min (Fig. 3D) with 30-min treatments (Fig. 3C). In contrast, the addition of isoxaben caused rapid clearing of YFP∷CESA6-labeled complexes from the plasma membrane focal plane, consistent with findings of Paredez et al. (2006). Thus, treatment with isoxaben or DCB resulted in opposite conditions at the plasma membrane.
The effect of plasmolysis on Arabidopsis seedlings expressing YFP∷CESA6 resulted in the formation of Hechtian strands decorated with the YFP∷CESA6 marker (Supplemental Fig. S3). Observation of YFP∷CESA6-labeled complexes remaining at the plant cell wall would be consistent with the model proposed by Lang et al. (2004) whereby Hechtian strands are tethered to the cellulose microfibril array via CESA rosettes in the plasma membrane. Pretreatment of Arabidopsis seedlings with DCB (Supplemental Fig. S2) or isoxaben (data not shown) prior to plasmolysis resulted in YFP∷CESA6-labeled complexes remaining localized to the protoplast during plasmolysis (Supplemental Fig. S2) with no observable CESA label left at the wall. Yet, using the plasma membrane marker PIP2∷GFP (Cutler et al., 2000), it was clear that under conditions of extended pretreatment (up to 3 h) with DCB or isoxaben, Hechtian strands were readily able to form, suggesting that their formation was independent of CESA rosettes (Supplemental Fig. S3). Several mechanisms have been proposed for Hechtian strand attachment to the plant cell wall involving plasmodesmata (Oparka et al., 1994), arabinogalactan proteins (Kohorn, 2001), wall-associated kinase (Kohorn, 2001), and Arg-Gly-Asp tripeptide sequences (Gouget et al., 2006). Based on our data, it is unlikely that CESA is solely responsible for Hechtian strand attachment to the cell wall.
Recent studies have demonstrated a functional association between CESA complexes and individual elements of the cortical microtubule array (Paredez et al., 2006; DeBolt et al., 2007). Therefore, we examined the effects of DCB on cortical microtubules using plants expressing YFP∷TUA5 or GFP∷MAP4. DCB caused no observable changes in microtubule dynamics or alignment in hypocotyl cells when compared with mock treatment during the 5 h following treatment (results not presented). Furthermore, DCB did not noticeably disrupt the morphology or motility of the actin cytoskeleton or endoplasmic reticulum, as observed using fluorescent protein markers, suggesting that changes in cell growth anisotropy caused by DCB did not result from gross cell toxicity (data not presented).
Results presented here concerning the effects of DCB on CESA suggest that DCB may be useful to elucidate the mechanism of CESA mobility and its insertion at the plasma membrane. In addition, these studies raise the possibility that DCB may be a useful tool for future efforts to purify CESA complexes due to enrichment at the membrane and apparent release of anchoring to the wall. In previous experiments, the density and bidirectional motility of CESA complexes have restricted the ability to faithfully follow the fate of individual complexes in a crowded environment (Paredez et al., 2006; DeBolt et al., 2007). Analysis of chromophore intensity profiles at the plasma membrane indicated that DCB caused large increases in emission at punctate sites over 150 min, suggesting a nonrandom pattern of CESA insertion or accumulation in the plasma membrane. These data are consistent with studies by Herth (1987), who showed an increase in the number of “intact” CESA rosettes in wheat roots after DCB addition. An alternative hypothesis for our observations is that after delivery to the membrane, complexes may move a short distance to the observed accumulation sites or CESA may have altered organization in these localized regions (i.e. more CESA6 subunits within multimeric rosettes). In our previous work (DeBolt et al., 2007), when microtubules were depleted using oryzalin, YFP∷CESA6 showed the same displacement velocity as when microtubules were present, demonstrating that microtubule motors such as kinesins are unlikely to be the engine for CESA motility; rather, CESA movement is likely propelled by the polymerization force of cellulose synthesis. Thus, results presented here imply that DCB and isoxaben, the two model inhibitors of cellulose biosynthesis in plants, cause different YFP∷CESA6 accumulation patterns due to different modes of action.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Deposition of callose in response to isoxaben and DCB is controlled by PMR4.
Supplemental Figure S2. Exogenous addition of SSG (21 μm) for 1 h after pretreatment with DCB (5 μm) for 2 h did not stimulate motility of YFP∷CESA6.
Supplemental Figure S3. Plasmolysis of mock- and DCB-treated cells shows that CESA complexes remain in the plasma membrane after DCB treatment and that Hechtian strand formation occurs independent of DCB treatment.
Supplemental Movie S1. Cessation of YFP∷CESA6 label upon DCB treatment.
Supplemental Materials and Methods S1. Description of all experimental procedures.
Supplementary Material
Acknowledgments
We thank S. Somerville, C. Bejar, and two anonymous reviews for comments.
This work was supported in part by grants from the U.S. Department of Energy (DOE–FG02–03ER20133) and the National Science Foundation (NSF–0524334).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Chris Somerville (crs@stanford.edu).
The online version of this article contains Web-only data.
References
- Anderson JR, Barnes WS, Bedinger P (2002) 2,6-Dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J Plant Physiol 159 61–67 [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant-tissue. Protoplasma 146 133–142 [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S, Gutierrez R, Ehrhardt DW, Melo CV, Ross L, Cutler SR, Somerville C, Bonetta D (2007) Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc Natl Acad Sci USA 104 5854–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Vernhettes S, Fagard M, Refrégier G, Desnos T, Aletti E, Py N, Pelletier S, Höfte H (2002) Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol 128 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann HG, Fry SC (1992) Effect of cellulose synthesis inhibition on growth and the integration of xyloglucan into pea internode cell walls. Plant Physiol 100 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, Pont-Lezica RP, Canut H (2006) Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol 140 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim DR, Skomp JR, Tschabold EE, Larrinua IM (1990) Isoxaben inhibits the synthesis of acid insoluble cell-wall materials in Arabidopsis thaliana. Plant Physiol 93 695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth W (1987) Effects of 2,6-DCB on plasma membrane rosettes of wheat root cells. Naturwissenschaften 74 556–557 [Google Scholar]
- Himmelspach R, Williamson RE, Wasteneys GO (2003) Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J 36 565–575 [DOI] [PubMed] [Google Scholar]
- Hogetsu T, Shibaoka H, Shimokor M (1974) Involvement of cellulose synthesis in actions of gibberellin and kinetin on cell expansion—2,6-dichlorobenzonitrile as a new cellulose synthesis inhibitor. Plant Cell Physiol 15 389–393 [Google Scholar]
- Kohorn BD (2001) WAKs; cell wall associated kinases. Curr Opin Cell Biol 13 529–533 [DOI] [PubMed] [Google Scholar]
- Lang I, Barton DA, Overall RL (2004) Membrane-wall attachments in plasmolysed plant cells. Protoplasma 224 231–243 [DOI] [PubMed] [Google Scholar]
- Lukowitz WR, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR (2001) Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to the requirement for glycosylation in cellulose biosynthesis. Proc Natl Acad Sci USA 98 2262–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezinos D, Delmer DP (1980) Characterization of inhibitors of cellulose synthesis in cotton fibers. Planta 148 305–311 [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Sakurai N (1998) Increase in the amount of celA1 protein in tobacco BY-2 cells by a cellulose biosynthesis inhibitor, 2,6-dichlorobenzonitrile. Plant Cell Physiol 39 779–785 [DOI] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301 969–972 [DOI] [PubMed] [Google Scholar]
- Ohmiya Y, Nakai T, Park YW, Aoyama T, Oka A, Sakai F, Hayashi T (2003) The role of PopCel1 and PopCel2 in poplar leaf growth and cellulose biosynthesis. Plant J 33 1087–1097 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Prior DAM, Crawford JW (1994) Behavior of plasma membrane, cortical ER and plasmodesmata during plasmolysis of onion epidermal cells. Plant Cell Environ 17 163–171 [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312 1491–1495 [DOI] [PubMed] [Google Scholar]
- Peng LC, Kawagoe Y, Hogan P, Delmer D (2002) Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 295 147–150 [DOI] [PubMed] [Google Scholar]
- Sabba RP, Durso NA, Vaughn KC (1999) Structural and immunocytochemical characterization of the walls of dichlobenil-habituated BY-2 tobacco cells. Int J Plant Sci 160 275–290 [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville CR (2001) Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc Natl Acad Sci USA 98 10079–10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedletzky E, Shmuel M, Trainin T, Kalman S, Delmer D (1992) Cell wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile: a comparison between two dicotyledonous plants and a Graminaceous monocot. Plant Physiol 100 120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22 53–78 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ingold E, Sugiyama M, Fukuda H, Komamine A (1992) Effects of 2,6-dichlorobenzonitrile on differentiation to tracheary elements of isolated mesophyll cells of Zinnia elegans and formation of secondary cell walls. Physiol Plant 86 43–48 [Google Scholar]
- Talbot MJ, Wasteneys GO, Offler CE, McCurdy DW (2007) Cellulose synthesis is required for deposition of reticulate wall ingrowths in transfer cells. Protoplasma 48 147–158 [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Hoffman JC, Hahn MG, Staehelin LA (1996) The herbicide dichlobenil disrupts cell plate formation: immunogold characterization. Protoplasma 194 117–132 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.