Abstract
Phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) plays a key role during C4 photosynthesis and is involved in anaplerotic metabolism, pH regulation, and stomatal opening. Heterozygous (Pp) and homozygous (pp) forms of a PEPC-deficient mutant of the C4 dicot Amaranthus edulis were used to study the effect of reduced PEPC activity on CO2 assimilation rates, stomatal conductance, and 13CO2 (Δ13C) and C18OO (Δ18O) isotope discrimination during leaf gas exchange. PEPC activity was reduced to 42% and 3% and the rates of CO2 assimilation in air dropped to 78% and 10% of the wild-type values in the Pp and pp mutants, respectively. Stomatal conductance in air (531 μbar CO2) was similar in the wild-type and Pp mutant but the pp mutant had only 41% of the wild-type steady-state conductance under white light and the stomata opened more slowly in response to increased light or reduced CO2 partial pressure, suggesting that the C4 PEPC isoform plays an essential role in stomatal opening. There was little difference in Δ13C between the Pp mutant (3.0‰ ± 0.4‰) and wild type (3.3‰ ± 0.4‰), indicating that leakiness (φ), the ratio of CO2 leak rate out of the bundle sheath to the rate of CO2 supply by the C4 cycle, a measure of the coordination of C4 photosynthesis, was not affected by a 60% reduction in PEPC activity. In the pp mutant Δ13C was 16‰ ± 3.2‰, indicative of direct CO2 fixation by Rubisco in the bundle sheath at ambient CO2 partial pressure. Δ18O measurements indicated that the extent of isotopic equilibrium between leaf water and the CO2 at the site of oxygen exchange (θ) was low (0.6) in the wild-type and Pp mutant but increased to 0.9 in the pp mutant. We conclude that in vitro carbonic anhydrase activity overestimated θ as compared to values determined from Δ18O in wild-type plants.
The enzyme phosphoenolpyruvate (PEP) carboxylase (PEPC) utilizes bicarbonate (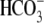 ) to catalyze the β-carboxylation of PEP, to form the four-carbon acid oxaloacetate (Andreo et al., 1987; Chollet et al., 1996; Lepiniec et al., 2003; Izui et al., 2004). In higher plants, PEPC plays the anaplerotic role of replenishing the citric acid cycle intermediates, oxaloacetate and malate, which are required for nitrogen assimilation and amino acid biosynthesis. The synthesis of malate through PEPC activity has also been implicated in cytosolic pH regulation (Lepiniec et al., 1994; Britto and Kronzucker, 2005). In photosynthetic tissues, PEPC participates in the exchange of CO2 and water between a leaf and the atmosphere through its role in guard cell metabolism. Additionally, in C4 species PEPC catalyzes the first carboxylation reaction of C4 photosynthesis in the mesophyll cells (Kanai and Edwards, 1999).
) to catalyze the β-carboxylation of PEP, to form the four-carbon acid oxaloacetate (Andreo et al., 1987; Chollet et al., 1996; Lepiniec et al., 2003; Izui et al., 2004). In higher plants, PEPC plays the anaplerotic role of replenishing the citric acid cycle intermediates, oxaloacetate and malate, which are required for nitrogen assimilation and amino acid biosynthesis. The synthesis of malate through PEPC activity has also been implicated in cytosolic pH regulation (Lepiniec et al., 1994; Britto and Kronzucker, 2005). In photosynthetic tissues, PEPC participates in the exchange of CO2 and water between a leaf and the atmosphere through its role in guard cell metabolism. Additionally, in C4 species PEPC catalyzes the first carboxylation reaction of C4 photosynthesis in the mesophyll cells (Kanai and Edwards, 1999).
C4 plants generally have high PEPC activity in the cytosol of mesophyll cells, allowing for the accumulation of four-carbon acids that subsequently diffuse into the bundle sheath cells (BSCs) for decarboxylation (Kanai and Edwards, 1999; Furbank et al., 2000). The specialized biochemistry and leaf anatomy of C4 plants results in CO2 partial pressure (pCO2) around the site of Rubisco severalfold higher than current atmospheric levels, significantly reducing the rates of photorespiration (Hatch, 1987).
Theoretical models of 13CO2 isotope discrimination (Δ13C) during C4 photosynthesis have been developed that link Δ13C to the ratio of intercellular to ambient pCO2 and bundle sheath leakiness (φ) defined as the fraction of CO2 fixed by PEPC that subsequently leaks out of the BSCs and is not fixed by Rubisco (Farquhar, 1983). Leakiness is a measure of the efficiency of C4 photosynthesis and has been estimated with concurrent measurements of leaf gas exchange and Δ13C in a number of C4 species (Henderson et al., 1992). It has been shown that φ increases in transgenic Flaveria bidentis with reduced Rubisco content, demonstrating that the balance between C3 and C4 cycle activity influences φ (von Caemmerer et al., 1997a, 1997b; Cousins et al., 2006a). C18OO discrimination (Δ18O) during C4 photosynthesis, which is largely determined by the residence time of CO2 within a leaf and the number of hydration reactions per CO2 molecule, is influenced by changes in the carbonic anhydrase (CA) activity (Cousins et al., 2006b) and the capacities of the C4 and C3 cycles.
In higher plants, independent of the photosynthetic pathway, PEPC participates in guard cell metabolism. Stomatal opening is achieved through the accumulation of high levels of solutes in guard cell vacuoles. The accumulation of potassium ions requires anions (such as malate or chloride) to provide charge balance and to maintain the membrane potential. Malate produced via PEPC is believed to contribute substantially to the maintenance of the proton and charge balance in these cells during stomatal opening (Allaway, 1973; Outlaw and Lowry, 1977). The production of malate in guard cells is thought to be directly linked to carbon metabolism as PEP, the substrate for carboxylation, originates mainly from carbon skeletons derived from starch breakdown in the guard cell chloroplast (Vavasseur and Raghavendra, 2005). Additionally, the amount of PEPC in guard cells of C3 plants has been shown to be an order of magnitude greater than in mesophyll cells when expressed on a protein basis (Cotelle et al., 1999).
The concentration of malate inside guard cells correlates with stomatal aperture in epidermal strips but it was also shown that the influence of malate is dependent on the availability of chloride (van Kirk and Raschke, 1978; Willmer and Fricker, 1996). The role of PEPC in stomatal opening was also confirmed in epidermal strips of C3 plants using a PEPC inhibitor (Parvathi and Raghavendra, 1997; Asai et al., 2000). At the whole leaf level, the use of antisense and overexpression of PEPC in Solanum tuberosum also suggested that malate accumulation is involved in stomatal function (Gehlen et al., 1996). This showed that rates of stomatal opening increased in plants overexpressing PEPC and decreased in plants with reduced levels of PEPC. However, low PEPC levels had no effect on steady-state stomatal conductance and overexpression of PEPC had only a marginal effect (Gehlen et al., 1996).
Isotope analysis of atmospheric carbon CO2 has become an important tool for monitoring changes in the global exchange of CO2 (Flanagan and Ehleringer, 1998; Yakir and Sternberg, 2000). However, to interpret the atmospheric CO2 isotopic signature requires an understanding of the isotopic fractionation steps associated with specific processes during leaf gas exchange (Yakir and Sternberg, 2000). Here we used the PEPC-deficient mutants of the C4 dicot Amaranthus edulis (Dever et al., 1995, 1996, 1997) to asses the contribution of PEPC activity on photosynthetic isotope exchange and stomatal conductance. Previous work on these plants has shown that the heterozygous (Pp) and the homozygous (pp) PEPC mutants contain approximately 50% and 2% of wild-type PEPC activity, respectively (Dever et al., 1996, 1997; Maroco et al., 1997, 1998a, 1998b; Kiirats et al., 2002). These mutants are a nice comparison to earlier isotope work on the C4 dicot F. bidentis that had high rates of PEPC and low CA due to antisense silencing of CA (Cousins et al., 2006a, 2006b). We have used the PEPC-deficient A. edulis mutants to address three distinct questions. (1) How does a reduction in PEPC activity affect Δ13C and bundle sheath leakiness? (2) Does the increased ratio of CA to PEPC activity affect the isotopic equilibrium between leaf water and CO2 and hence Δ18O? (3) What are the effects of reduced PEPC activity on stomatal conductance?
RESULTS
Steady-State Gas-Exchange and Enzyme Activities
Under our growth conditions, which contained 9.8 mbar of CO2, both the heterozygous (Pp) and homozygous (pp) PEPC mutants had similar total nitrogen per leaf area and leaf mass per area as compared to wild-type plants (Table I). Concurrent measurements of Δ13C and Δ18O and gas exchange were made by directly coupling a mass spectrometer to the outlet of a portable leaf gas-exchange system via a gas permeable silicone membrane (Cousins et al., 2006a, 2006b). This allowed simultaneous measurements of leaf gas exchange and the 13C/12C or 18O/16O ratios of the CO2 in the air stream without prior purification of the CO2. Under ambient CO2 concentrations (531 μbar), net CO2 assimilation rates in the Pp and pp mutants were 78% and 10% of wild-type plants, respectively (Table I). Compared to the wild type, the ratio of intercellular to atmospheric pCO2 (pi/pa) was higher and stomatal conductance (gs) lower in the pp mutant at high light and ambient CO2 (531 μbar), whereas the differences between the wild-type and Pp mutant were not significant. PEPC activity determined on whole leaf extracts was 42% and 3% of wild-type in Pp and pp mutants, respectively, whereas Rubisco activity was not significantly different between the wild-type and pp mutant (Table I). The total extractable CA activity expressed as the rate constant kCA (μmol m−2 s−1 bar−1) was similar in all plants (Table I). The kCA was determined from leaf extracts using mass spectrometry to measure the rates of 18O2 exchange from labeled 13C18O2 to 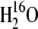 (Badger and Price, 1989; Cousins et al., 2006a, 2006b). Leaf CA activity (CAleaf), determined as the product of kCA and the mesophyll pCO2 (pm), increased in the pp mutant due to the low rates of net CO2 assimilation that caused pm to be greater in these plants (Table I). The values of pm were calculated as pm = pi − A/gm, where A is the net CO2 assimilation rate and gm is the CO2 conductance from the intercellular air space to the site of PEPC carboxylation (assumed to equal 1 mol m−2 s−1 bar−1; Cousins et al., 2006a).
(Badger and Price, 1989; Cousins et al., 2006a, 2006b). Leaf CA activity (CAleaf), determined as the product of kCA and the mesophyll pCO2 (pm), increased in the pp mutant due to the low rates of net CO2 assimilation that caused pm to be greater in these plants (Table I). The values of pm were calculated as pm = pi − A/gm, where A is the net CO2 assimilation rate and gm is the CO2 conductance from the intercellular air space to the site of PEPC carboxylation (assumed to equal 1 mol m−2 s−1 bar−1; Cousins et al., 2006a).
Table I.
Gas exchange and leaf characteristics of wild-type A. edulis and PEPC mutants
Net CO2 assimilation rate (A), the ratio of intercellular to ambient CO2 partial pressures (pi/pa), stomatal conductance (gs), carbon isotope discrimination (Δ13C), oxygen isotope discrimination (Δ18O), PEPC and Rubisco activity, the rate constant of leaf CA (kCA mol m−2 s−1 bar−1), leaf CA activity (CAleaf), the extent of isotopic equilibrium, leaf mass per area (LMA), and total leaf nitrogen per area in wild-type (WT) and heterozygous (Pp) and homozygous (pp) mutant A. edulis plants. Gas-exchange measurements were made at a pCO2 of 531 μbar, a pO2 of 48 mbar, irradiance of 2,000 μmol quanta m−2 s−1, and a leaf temperature of 30°C. Calculations of θ were made with gm = 1 mol m−2 s−1 bar−1. Shown are the means ± se of measurements made on three to five leaves from separate plants. nd indicates measurements were not determined. Statistical analysis was conducted using an ANOVA and different letters indicate significant differences between plants at P < 0.05. *, CA leaf = kCApm and pm was calculated from A and pi using a conductance of CO2 from the intercellular air space to the site of PEPC carboxylation equal to 1 mol m−2 s−1 bar−1.
| Measurements | WT | Pp | pp |
|---|---|---|---|
| Photosynthetic parameters | |||
| A (μmol m−2 s−1) | 40.9 ± 1.6a | 32.1 ± 1.7b | 4.1 ± 0.4c |
| pi/pa | 0.39 ± 0.06a | 0.45 ± 0.07a | 0.82 ± 0.02b |
| gs (mol m−2 s−1) | 0.28 ± 0.04a | 0.25 ± 0.06a | 0.09 ± 0.01b |
| Δ13C ‰ | 3.3 ± 0.4a | 3.0 ± 0.5a | 16.2 ± 3.0b |
| Δ18O ‰ | 16.8 ± 1.8a | 20.9 ± 3.1a | 207.4 ± 50.8b |
| Enzyme activities | |||
| PEPC (μmol m−2 s−1) | 144.9 ± 8.8a | 61.0 ± 4.6b | 3.8 ± 0.4c |
| Rubisco (μmol m−2 s−1) | 44.2 ± 3.2a | nd | 48.1 ± 3.2a |
| kCA (mol m−2 s−1 bar−1) | 6.1 ± 0.4a | 5.3 ± 0.7a | 6.4 ± 1.0a |
| CAleaf* (μmol m−2 s−1) | 783 ± 153a | 831 ± 113a | 2818 ± 64b |
| Isotopic equilibrium (θ) | |||
| Predicted (Eq. 12) | 0.98 ± 0.01a | 0.98 ± 0.01a | 1.00 ± 0.00a |
| Measured (Eq. 11) | 0.62 ± 0.02a | 0.62 ± 0.07a | 0.91 ± 0.05b |
| Leaf parameters | |||
| Total leaf N (mmol m−2) | 132 ± 23a | 120 ± 10a | 130 ± 4a |
| LMA (g m−2) | 34.0 ± 2.9a | 38.5 ± 3.2a | 30.8 ± 1.6a |
13CO2 and C18OO Discrimination
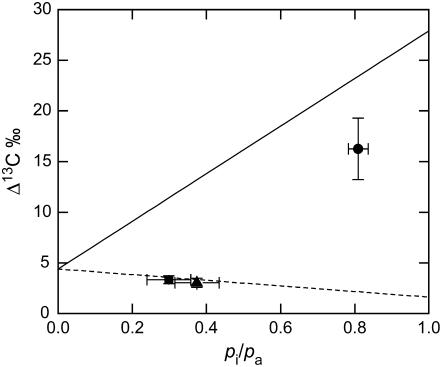
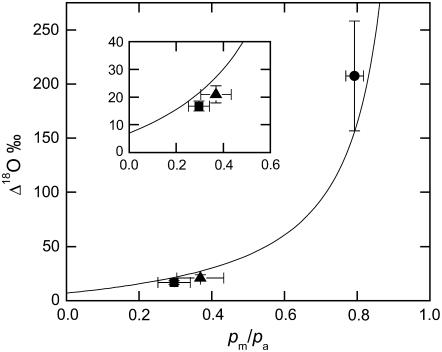
Carbon isotope discrimination (Δ13C) decreased slightly but not significantly in the Pp mutant as compared with the wild type (Table I). In the pp mutant the value of Δ13C was approximately 5-fold higher than in wild-type and Pp plants, and pi/pa was 0.82 compared to 0.39 in the wild type (Table I; Fig. 1). The values of Δ18O were not significantly different between the wild-type and the Pp plants but Δ18O was 12-times higher in the pp mutant compared to the wild type (Table I; Fig. 2). The proportion of CO2 in isotopic equilibrium with water at the site of oxygen exchange (θ) predicted from in vitro CA assays (Eq. 12) was substantially higher in the wild-type and Pp plants than the θ values estimated from Δ18O measurements (Eq. 11; Table I). However, θ calculated from in vitro CA assays (Eq. 12) and Δ18O measurements (Eq. 11) were similar in the pp mutant (Table I). The Δ18O increased with pm/pa as predicted from Equation 8, but the measured values of Δ18O were less than those predicted at full isotopic equilibrium for the wild-type and Pp mutant (Fig. 2). The pp mutant had a high pm/pa but wild-type levels of extractible CA activity and the measured values of Δ18O were closer to the predicted values of Δ18O, compared to wild-type plants (Table I; Fig. 2). The theoretical line of full isotopic equilibrium in Figure 2 was calculated using Equation 8, assuming an average value of the oxygen isotope composition of CO2 at the site of exchange during photosynthesis (Δea), taken from all plants in Table II, of 39.2‰. The values of Δea were not significantly different between the plants (Table II).
Figure 1.
Carbon isotope discrimination (Δ13C) as a function of the ratio of intercellular to ambient pCO2 (pi/pa) in wild-type and mutant A. edulis plants. The dashed line represents the theoretical relationship of Δ13C4 and pi/pa during C4 photosynthesis where φ = 0.24, using Equation 6. The solid lines represent the theoretical relationship of Δ13C3 and pi/pa using the C3 model (Eq. 3). Gas-exchange conditions are as in Table I. Each point represents the means ± se of measurements made on three to five leaves from separate plants from wild type (▪), Pp mutant (▴), and pp mutant (•).
Figure 2.
Oxygen isotope discrimination (Δ18O) as a function of the ratio of mesophyll cytosolic to ambient CO2 partial pressure (pm/pa). pm was calculated with gm = 1 mol m−2 s−1 bar−1. The line represents the theoretical relationship of Δ18O and pm/pa at full isotopic equilibrium where a = 7.7‰ and Δea = 33.7‰ (Eq. 8) and the CO2 supplied to the leaf had a δ18O of 24‰ relative to VSMOW. Symbols are as in Figure 1 and measurement conditions are as in Table I. The inset shows the expanded scale of Δ18O for the wild-type and Pp plants.
Table II.
Oxygen isotope exchange parameters of wild-type A. edulis and PEPC mutants
The δ18O of water at the site of evaporation (δe), 18O enrichment of CO2 compared to the atmosphere at the site of exchange in full oxygen isotope equilibrium (Δca), the oxygen isotope composition of CO2 at the site of exchange during photosynthesis (Δea), the ratio of the vapor pressure in the atmosphere to the leaf intercellular spaces (ea/ei), the residence time of CO2 in the aqueous phase within the leaf (τ s−1), and the intercellular pCO2 (pi) in wild-type (WT) and heterozygous (Pp) and homozygous (pp) mutant A. edulis plants. Source water was −5.3 ± 0.3. Measurement conditions as in Table I. Shown are the means ± se of measurements made on three to five leaves from separate plants. Statistical analysis was conducted using an ANOVA and different letters indicate significant differences between plants at P < 0.05.
| Plants | δe | Δca | Δea | ea/ei | τ s−1 | pi |
|---|---|---|---|---|---|---|
| ‰ | ‰ | ‰ | ||||
| WT | 24.0 ± 0.8a | 20.6 ± 0.6a | 36.9 ± 1.4a | 0.41 ± 0.03a | 1.9 ± 0.1a | 166 ± 19a |
| Pp | 24.7 ± 0.9a | 21.6 ± 3.0a | 37.6 ± 1.5a | 0.39 ± 0.03a | 2.8 ± 0.2a | 199 ± 29a |
| pp | 29.9 ± 0.9b | 38.8 ± 1.1b | 43.2 ± 1.7a | 0.21 ± 0.03b | 16.5 ± 2.7b | 431 ± 14b |
The δ18O of water at the site of evaporation (δe) was similar in the wild-type and Pp plants but significantly more enriched in the pp plants (Table II). The 18O enrichment of CO2 compared to the atmosphere at the site of exchange in full oxygen isotope equilibrium (Δca) and the ratio of the water vapor pressure in the atmosphere to the leaf intercellular spaces (ea/ei) were also similar in the wild-type and Pp plants but were different in the pp plants (Table II). The residence time of CO2 in the aqueous phase within the leaf (τ = pm/Fin) and the intercellular pCO2 were greater in the pp plants compared to the wild-type and Pp plants (Table II).
Stomatal Response
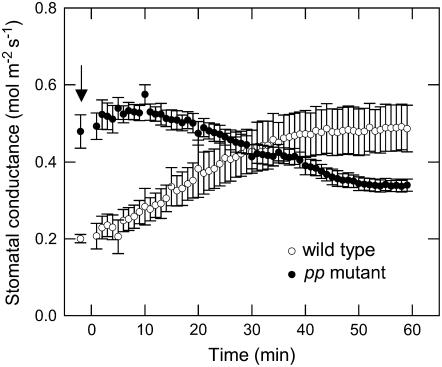
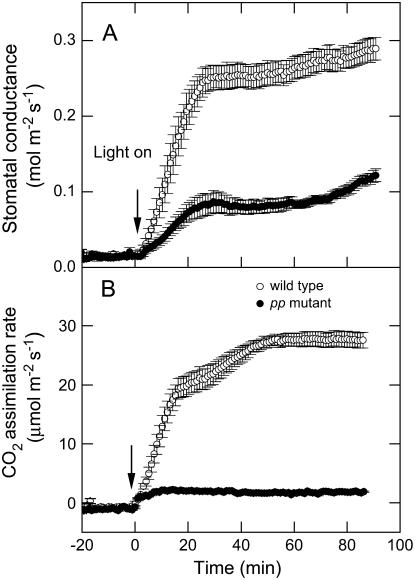
Online measurements of Δ13C and Δ18O leaf gas exchange at 531 μbar CO2 partial pressure indicated that steady-state leaf conductance in the homozygous pp mutant was reduced compared to the wild type under these conditions (Table I). Further analysis showed that stomatal conductance (gs) under growth conditions (9.8 mbar CO2, 400 μmol quanta m−2 s−1, air humidity 29–32 mmol mol−1, leaf temperature of 30°C) was higher in the pp mutant (0.7 ± 0.1 mol m−2 s−1) compared to wild type (0.3 ± 0.1 mol m−2 s−1; Fig. 3). However, gs declined in the pp mutant and increased in the wild type when plants were rapidly shifted from growth conditions to similar conditions with low CO2 (364 μbar; Fig. 3). To compare the response of gs under laboratory conditions the pp and wild-type plants were dark adapted overnight in ambient CO2 (364 μbar) and gas exchange was measured the following day. The two genotypes showed no difference in gs under steady-state conditions in the dark before the onset of illumination (Fig. 4). However, the homozygous pp mutant had an approximately 3-times lower rate of stomatal opening (Fig. 4) in response to light under 364 μbar CO2 than the wild type and gs after 90 min in the light was only 41% of wild-type values (Fig. 4). In spite of the difference in steady-state conductance, both the wild-type and the pp mutant reached half their maximal conductance within approximately 13 min of the onset of illumination (Fig. 4). Long-term (5 h) measurements of gs under the same conditions showed that the difference in stomatal conductance between the wild-type and pp mutant plants was maintained (data not shown).
Figure 3.
Stomatal conductance for wild-type and pp mutant plants under growth conditions (indicated by arrow) at 400 μmol quanta m−2 s−1, leaf temperature of 30°C, and the leaf chamber humidity was 29.6 ± 0.6 and 32.4 ± 0.4 mmol mol−1 for the wild-type and pp mutant, respectively. Plants were subsequently transferred from the growth cabinets at time zero and a leaf was immediately placed into the gas-exchange chamber under growth conditions except the CO2 concentration was 360 μbar instead of 9.8 mbar. The leaf chamber humidity was maintained at 30.01 ± 0.01 for both the wild-type and pp mutant. Data are the means of measurements of four different wild-type and five different pp mutants, error bars represent se; wild type (○) and pp mutant (•).
Figure 4.
Light induction of stomatal conductance (A) and net CO2 assimilation (B) in wild-type and the pp mutant. Plants were grown under 9.8 mbar CO2 and then transferred to ambient CO2 in the dark overnight. Leaf gas exchange was measured for several minutes prior to the start of illumination at 2,000 μmol quanta m−2 s−1 (indicated by the arrow). The rate of stomatal opening for the wild-type and pp mutant was 7.8 ± 1.9 and 2.4 ± 0.5 (mmol water m−2 s−2), respectively. The ambient CO2 partial pressure was maintained at 364 μbar for the duration of the measurements. Data are the means of measurements of six different plants, error bars represent se; wild type (○) and pp mutant (•).
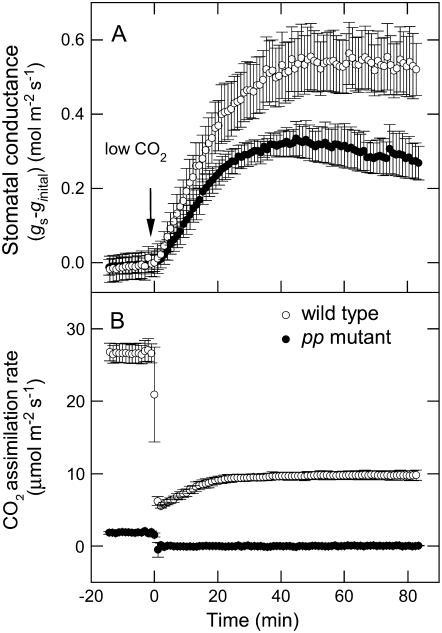
To examine whether the guard cells in the pp mutant were sensitive to changes in pCO2, leaf gas exchange was measured under steady-state conditions (364 μbar CO2, 2,000 μmol quanta m−2 s−1 and a vapor-pressure difference of 10 mbar) and then the pCO2 was dropped to 48 μbar. Compared to the wild type, the pp mutant had a lower initial rate of stomatal opening and steady-state conductance reached only half of wild-type values (Fig. 5). As in the response to light, the halftime of stomatal opening to CO2 was similar in both types of plants (14.1 ± 0.9 min and 11.2 ± 0.7 min, for wild-type and pp mutant, respectively; Fig. 5).
Figure 5.
A, The net change in stomatal conductance over time after a step change from 364 μbar to 48 μbar CO2, normalized by the initial conductance in 364 μbar CO2. Steady-state conductance at low CO2 was 0.83 ± 0.08 (wild type) and 0.41 ± 0.05 (pp mutant) mol water m−2 s−1 and the rate of stomatal opening for the wild-type and pp mutant was 20.2 ± 4.5 and 11.9 ± 2.3 (mmol water m−2 s−2), respectively. Illumination was kept at 2,000 μmol quanta m−2 s−1 for the duration of the experiment. B, The change in CO2 assimilation rate over time at low CO2 partial pressure is shown for comparison. Data are the means of measurements of four and five different plants, respectively, for the wild-type (○) and homozygous pp mutant (•). Error bars represent se.
Epidermal PEPC Content and Stomatal Density
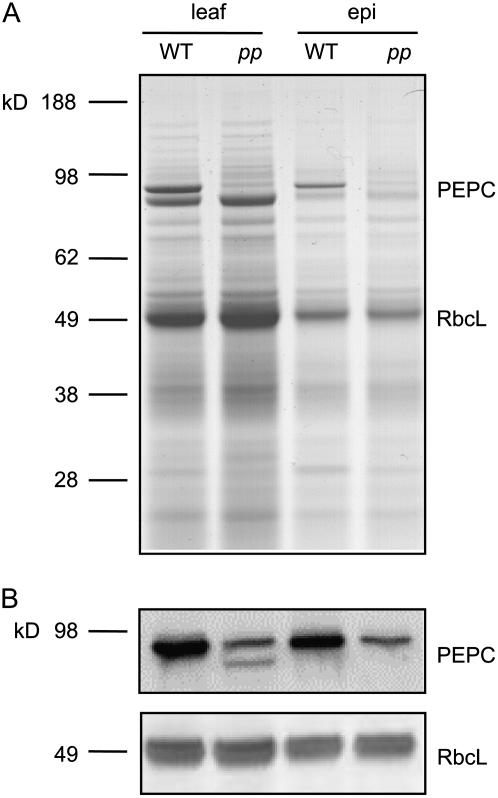
In agreement with previous reports (Dever et al., 1995, 1996, 1997; Maroco et al., 1998b) and our activity measurements (Table I), leaf tissue of the pp mutant showed a large decrease in the content of PEPC, detected either by Coomassie staining or by immunoblot (Fig. 6). An epidermal fraction showed a protein profile similar to that of whole leaves and was identical in wild-type and pp mutant with the exception of the band corresponding to PEPC. The pp mutant epidermis contained 53% ± 4% of wild-type PEPC as determined by western analysis on three wild-type and four pp mutant plants (Fig. 6 shows one such representative western). The number of stomata per unit leaf area was greater in the pp mutant (63% and 77% greater than in the wild type on the adaxial and abaxial sides, respectively); however, the stomatal index remained unchanged (Table III).
Figure 6.
Soluble protein profile of leaf discs and epidermis of A. edulis wild-type (WT) and pp mutant. A, Coomassie Blue-stained SDS-PAGE gel of soluble leaf protein from leaf and epidermal (epi) fraction. B, Immunoblot of PEPC and the large subunit of Rubisco (RbcL), shown as a loading control. Thirty micrograms of total protein were loaded per lane. The epidermal PEPC protein content of the pp mutant was 53% ± 4% of wild type. The relative abundance of epidermal PEPC protein in the pp mutant compared to wild type was determined from immunoblot labeling of three wild-type and four pp mutant epidermal extractions. Shown here is one representative blot.
Table III.
Stomatal density and index of wild-type A. edulis and PEPC pp mutants
Stomatal index and stomatal density per leaf area in wild-type and homozygous mutant A. edulis. Shown are the means ± se of counts taken on leaves from three (wild type) or four (pp mutant) plants. Statistical analysis was conducted using a Student's t test and different letters indicate significant differences between wild type (WT) and pp plants at P < 0.1.
| Leaf Side | Plant | Stomatal Density | Stomatal Index (Stomata/Total Epidermal Cells) |
|---|---|---|---|
| mm−2 | |||
| Adaxial | WT | 144 ± 11a | 0.22 ± 0.01a |
| pp | 233 ± 35b | 0.22 ± 0.01a | |
| Abaxial | WT | 123 ± 7a | 0.23 ± 0.01a |
| pp | 218 ± 35b | 0.22 ± 0.01a |
DISCUSSION
13CO2 Isotope Discrimination in the PEPC Mutants
The low activity of PEPC caused rates of net CO2 assimilation in the heterozygous (Pp) and the homozygous (pp) PEPC mutant to be significantly less than wild-type plants (Table I) when measured under ambient CO2 (531 μbar) concentrations as previously reported (Dever et al., 1997, 1998; Maroco et al., 1998a, 2000; Kiirats et al., 2002). Limited activity of PEPC during C4 photosynthesis causes a decrease in the initial CO2 carboxylation reaction and reduces the capacity of the C4 pump to concentrate CO2 within the BSCs (von Caemmerer, 2000; Cousins et al., 2006a). In the model of C4 carbon isotope discrimination (Eq. 6 in “Materials and Methods”) the main factors that influence Δ13C are changes in the intercellular to ambient CO2 partial pressures (pi/pa), the fraction of CO2 fixed by PEPC that subsequently leaks out of the BSC (φ), and the combined fractionation of PEPC and the isotopic equilibrium during dissolution of CO2 and conversion to bicarbonate (b4), which is dependent on the ratio of PEPC to CA activity (Farquhar, 1983). During C4 photosynthesis decreases in φ and b4 are both predicted to cause values of Δ13C to decrease (Farquhar, 1983). The values of Δ13C and pi/pa in the Pp mutant were not significantly different from the wild-type plants even though the Pp plants had lower rates of net CO2 assimilation (Table I). This implies that neither φ nor the b4 value was significantly different between wild-type and Pp plants. We conclude that although photosynthetic rates were lower in the Pp mutant compared to wild type, the Δ13C remained constant because the balance in the C3 and C4 cycles was not altered.
The very low PEPC activity in the pp plants (Table I) severely inhibited the initial carboxylation step of the C4 photosynthetic pathway causing the rates of net CO2 assimilation to decrease considerably relative to wild type (Table I; Fig. 4B). The value of pi/pa increased in the pp plants compared to wild type and the Pp plants (Table I) and according to the Δ13C model during C4 photosynthesis (Eq. 6) a decrease in pi/pa leads to a decrease in Δ13C at φ values of less than approximately 30% (Fig. 1). However, values of Δ13C were dramatically higher in the pp plant compared to the wild-type and Pp plants (Figs. 1) even though pi/pa was higher in the pp plants and the b4 would be close to −5.7 (Table I; Eq. 7). This implies that the simplistic model of C4 isotope exchange presented here does not provide an accurate description of the processes contribution to Δ13C in the pp plants (see below).
C3-Like Isotope Discrimination in the pp Mutant
As reported previously, the defective C4 cycle in the pp plants necessitates the direct diffusion of atmospheric CO2 into the BSC for CO2 assimilation (Dever et al., 1995, 1997; Maroco et al., 1998a, 1998b; Kiirats et al., 2002) and the instantaneous Δ13C in these plants is more accurately described by the Δ13C3 model for C3 photosynthesis with a low conductance of CO2 diffusion to the site of Rubisco carboxylation (gi) within the BSC (see below). Assuming that the discriminations associated with photorespiration and respiration are negligible (see Eq. 1), we estimated an average CO2 conductance from the intercellular air space to the site of Rubisco carboxylation within the BSC (gi) of 33 ± 10 mmol m−2 s−1 in the pp plants (Eq. 5). This value of gi is toward the high end of the range reported in the literature from various C4 plants and approximately 4-times higher than the gbs value reported by Kiirats et al. (2002) in their experiments with the pp plants. Mathematical modeling of the C4 photosynthetic pathway suggests that such high conductance values need to be matched with high biochemical capacity to maintain low values of leakiness (for discussion see von Caemmerer, 2003).
To estimate the effect of photorespiratory and respiratory fractionation on our estimates of gi we used discrimination factors for photorespiration (f = 10‰) and for respiration (e = −6‰) reported from the literature (Gillon and Griffiths, 1997; Ghashghaie et al., 2003; Igamberdiev et al., 2004). Our measurements were made at low O2 partial pressures so that the expected fractionation from photorespiration is only about 0.17‰ at a Γ* of 9.25 μbar. The effect of respiratory fractionation is between −1.67‰ to 1‰. It therefore appears that neither photorespiration nor respiration can easily account for the high Δ13C values measured in the pp plants and the major contributing factor to the high Δ13C values in these plants is the direct diffusion of CO2 from the intercellular air spaces to the site of Rubisco carboxylation with the BSC.
Determining absolute values of gi in C4 plants and BSC leakiness is difficult as these parameter are effected by numerous factors including growth and measurement conditions (Henderson et al., 1992; Cousins et al., 2006a; Kubásek et al., 2007). Potentially gi can be influenced by changes in the diffusivity of CO2 across cell walls, the cytoplasm in the mesophyll and BSC, and across plasma and chloroplast membranes (von Caemmerer and Furbank, 2003). Additionally, the BSC surface area to leaf area ratio can also influence gi; however, we saw no differences in leaf vein density in the pp plants (data not shown), indicating that the BSC surface area on a leaf area basis had not increased. However, because the pp plants rely on the direct diffusion of CO2 into the BSC to sustain photosynthesis, the internal conductance of CO2 may be greater than in wild-type plants, increasing the CO2 concentration within the BSC.
C18OO Isotope Discrimination and CO2/Water Isotopic Equilibrium
In a leaf, the oxygen isotope composition of CO2 is determined by the isotope composition of leaf water at the site of evaporation (δe) and CA activity. The exchange of 18O between CO2 and water is facilitated by CA, which catalyzes the interconversion of CO2 and bicarbonate (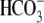 ), and high CA activity will increase the proportion of CO2 in isotopic equilibrium with the water. Based on calculated values of δe (Table II) the C18OO discrimination (Δ18O) was low in the wild-type plants compared to the high levels of leaf CA (CAleaf; Table I). The extent of isotopic equilibrium (θ) measured from Δ18O (Eq. 11) was also low in the wild-type plants relative to θ estimated from CA activity using Equation 12 (Table I). These findings are similar to previous work with another C4 dicot, F. bidentis, which also had low Δ18O and θ measured from Δ18O compared to the high rates of CAleaf (Cousins et al., 2006b). This further suggests that the total leaf CA activity in C4 dicots does not represent the CA activity associated with the CO2-water oxygen exchange that influences Δ18O (Cousins et al., 2006b).
), and high CA activity will increase the proportion of CO2 in isotopic equilibrium with the water. Based on calculated values of δe (Table II) the C18OO discrimination (Δ18O) was low in the wild-type plants compared to the high levels of leaf CA (CAleaf; Table I). The extent of isotopic equilibrium (θ) measured from Δ18O (Eq. 11) was also low in the wild-type plants relative to θ estimated from CA activity using Equation 12 (Table I). These findings are similar to previous work with another C4 dicot, F. bidentis, which also had low Δ18O and θ measured from Δ18O compared to the high rates of CAleaf (Cousins et al., 2006b). This further suggests that the total leaf CA activity in C4 dicots does not represent the CA activity associated with the CO2-water oxygen exchange that influences Δ18O (Cousins et al., 2006b).
The value of θ is related to the mean number of hydration reactions a CO2 molecule experiences inside a leaf. This in turn is the product of residence time (τ = pm/Fin) and the hydration constant of leaf CA (kCA), where pm is the mesophyll pCO2 and Fin is the gross flux of CO2 into the leaf (Eq. 12). The low photosynthetic rates increase the residence time of CO2 as pm increases (Tables I and II). Therefore, the number of hydration reactions per CO2 increases when rates of net CO2 assimilation are reduced by low PEPC activity. The amount of CA, expressed as the rate constant, was similar in the wild-type and PEPC mutants (Table I); however, under similar gas-exchange conditions the CAleaf activity in the PEPC mutants were higher (Table I). The increase in CAleaf in these plants is attributed to an increase in substrate availability for CA due to the lack of photosynthetic CO2 drawdown caused by the low PEPC activity. The increase in θ in the pp plants suggests that in wild-type A. edulis the CAleaf activity does not allow full isotopic equilibrium between the CO2 and water within the leaf under steady-state conditions.
Our study suggests that in C4 species leaf CA activity cannot readily be used as an indicator of the extent of 18O equilibration as has had been suggested by Gillon and Yakir (2001). This might be because not all of the CA located in mesophyll cytosol in C4 species is available at the site of CO2 water exchange compared to C3 species where CA is located in chloroplasts that appress intercellular airspaces (Poincelot, 1972; Ku and Edwards, 1975). Alternatively, the extent of 18O equilibration may be miscalculated if the estimated values of δe do not accurately describe the isotopic composition of water at the site of oxygen exchange between leaf water and CO2.
Stomatal Conductance in Response to Light and Atmospheric CO2
The pp mutant had low stomatal conductance (gs) during steady-state gas-exchange conditions (at 531 μbar CO2) relative to the Pp mutant and wild-type plants (Table I). Additionally, gs in the pp mutant decreased but increased in the wild type when leaves were rapidly transferred from the high CO2 (9.8 mbar) growth conditions into air (364 μbar CO2) at a constant leaf chamber humidity (Fig. 3). The higher gs under elevated CO2 reported here is consistent with previous reports that gs is generally greater under superelevated CO2 (above 4.0 mbar) compared to air CO2 concentrations (see review and references within Wheeler et al., 1999). Low gs in the pp mutant at ambient CO2 (364 or 531 μbar) is different from previous publications where stomatal conductance was generally not affected when the photosynthetic capacity was reduced due to antisense silencing of either Rubisco or Rubisco activase in the C4 dicot F. bidentis (von Caemmerer et al., 1997b, 2005). In C4 plants PEPC initiates carbon fixation in mesophyll cells but also plays an important role in providing malate as a counter ion and an osmoregulator in the guard cells to help counterbalance the large influx of potassium ions during stomatal opening (Vavasseur and Raghavendra, 2005). The content of PEPC in the pp mutant was reduced at both the whole leaf level as well as in the epidermal tissue, indicating that the C4 PEPC gene is the same as the guard cell gene in A. edulis (Table I; Fig. 6). Therefore, reduced PEPC activity in the pp mutant not only limited photosynthetic rates but likely impaired the accumulation of malate in the guard cells. Studies with epidermal peels have demonstrated strong correlations between stomatal opening and malate accumulation in guard cells (Allaway, 1973; Pearson, 1973; van Kirk and Raschke, 1978) and with the use of a PEPC inhibitor (DCDP) it has also been shown that stomatal opening is restricted when PEPC activity is reduced in epidermal strips (Parvathi and Raghavendra, 1997). In epidermal strips, the importance of malate as a counter ion to K+ is influenced by the availability of chloride (van Kirk and Raschke, 1978; Schnabl and Raschke, 1980; Willmer and Fricker, 1996). The low gs and epidermal PEPC content in the pp mutants show that maximum stomatal opening is dependent on PEPC activity and presumably malate in the guard cells in vivo.
In the dark gs was similar in pp mutant and wild type at air CO2 concentrations (364 μbar), but the rate of opening during the light induction was slow in the pp mutant and the stomata were able to maintain only a third of the conductance under steady-state conditions compared to wild-type plants (Fig. 4). These findings provide further support that PEPC is necessary for stomatal opening in response to light (Asai et al., 2000). Interestingly, both the wild-type and pp mutants reached half their maximal gs rate at similar times (Fig. 4), indicating that the perception of changing light conditions was not inhibited in the pp mutant, only that the stomata could not open as quickly and were unable to establish high rates of conductance.
Stomatal conductance increased in both the wild-type and the pp mutant in response to lowering pCO2 (Fig. 5). However, gs was slower to respond to the shift in CO2 in the pp mutants and did not reach similar rates as in the wild-type plants (Fig. 5). The pp mutant can therefore sense the change in CO2 availability but lacks the ability to achieve maximal values of gs in response to conditions that normally stimulate gs. As with the light response, even though gs in the pp mutant did not reach similar values to the wild-type plants, the values of gs increased about three times in response to low CO2 availability in both plants (Fig. 5).
The shifts in gs in response to changing light and pCO2 did not correlate with the changes in net CO2 assimilation in the pp plants (Fig. 5). For example, there was only a slight increase in net CO2 assimilation from the dark to light transition in the pp plants but stomatal conductance increased about 8 times (Fig. 4). This increase in gs during the light induction was less than in the wild-type plants but was still significant. Changes in net CO2 assimilation were also minor in response to CO2 in the pp plants (Fig. 5) but gs was approximately 3-times greater under the lower CO2 concentrations (Fig. 5). Although it has been demonstrated that there is a tight correlation between gs and photosynthetic capacity in both C3 and C4 plants (Wong et al., 1985), the use of antisense and photosynthetic mutants indicates that under certain conditions this relationship may not hold (for review, see von Caemmerer et al., 2004a).
The low gs in the pp mutant could have been attributed to reduced stomatal density compared to wild-type plants; however, stomatal density was approximately 1.5-times greater in the pp mutant, both adaxial and abaxial, than in the wild type (Table III). In fact stomatal conductance was higher in the pp mutant compared to the wild type under the 9.8 mbar CO2 growth conditions (Fig. 3), which may in part be due to the alleviation of PEPC limitation on gs in the pp mutant by high CO2 availability coupled with the higher stomatal density in the pp mutant (Table II). The stomatal index in these two plants was similar, indicating that the increase in stomata in the pp plants was due to a general increase in the number of total epidermal cells (Table III). The increase in stomatal density may help alleviate the BSC CO2 limitation in the pp plants that rely on direct fixation of atmospheric CO2 by Rubisco.
CONCLUSION
The reduction in PEPC activity in A. edulis reduced rates of net CO2 assimilation and Δ13C and Δ18O were dramatically increased in the homozygous PEPC mutant (pp). The high Δ13C value in the pp plants is likely caused by the direct diffusion of CO2 from the intercellular air spaces to the site of Rubisco carboxylation within the BSC. The isotopic equilibrium between leaf water and the intercellular pCO2 appears to be overestimated by in vitro measurements of total leaf CA activity compared to isotopic equilibrium determined from Δ18O measurements in wild-type plants. Lower stomatal conductance under steady-state conditions and the slower responses of stomata to changing light and CO2 conditions in the pp mutant corresponded with reduced PEPC content in the epidermal tissue, implicating the C4 isoform of PEPC in controlling stomatal movement.
MATERIALS AND METHODS
Growth Conditions
Seeds from the F2 population of Amaranthus edulis LaC4 2.16 mutant deficient in PEPC activity (Dever et al., 1995, 1997) and from the corresponding wild type were grown under 9.8 mbar of CO2 in a controlled environment growth cabinet at an irradiance of 400 μmol quanta m−2 s−1 at plant height and air temperature of 27°C during the day and 18°C at night, with a day length of 14 h. Plants were grown in 5 L pots in garden mix with 2.4 to 4 g Osmocote/L soil (15/4.8/10.8/1.2 N/P/K/Mg + trace elements: B, Cu, Fe, Mn, Mo, Zn; Scotts Australia Pty Ltd.) and watered daily. The mutant plants were screened by gas exchange and PEPC activity (see below).
Gas-Exchange Measurements
Online 13CO2 and C18OO Discrimination
The uppermost fully expanded leaves were placed into the leaf chamber of the LI-6400 portable gas-exchange system (LI-COR) and equilibrated under measurement conditions for a minimum of 1.5 h (Cousins et al., 2006a, 2006b). Air entering the leaf chamber was prepared by using mass flow controllers (MKS instruments) to obtain a gas mix of 909 mbar dry N2 and 48 μbar O2. A portion of the nitrogen/oxygen air was used to zero the mass spectrometer to correct for N2O and other contaminants contributing to the 44, 45, and 46 peaks. Pure CO2 (δ13CVPDB = −29‰, and δ18OVSMOW = 24‰) was added to the remaining air stream to obtain a CO2 partial pressure of approximately 531 μbar. The isotopic CO2 used for measurements was similar to that used in the controlled environments. Low oxygen (48 mbar) was used to minimize contamination of the 46 (mass-to-charge ratio) signal caused by the interaction of O2 and N2 to produce NO2 with the mass spectrometer source. Simultaneous measurements of leaf gas-exchange and isotope discrimination were determined by linking the LI-6400 gas-exchange system to a mass spectrometer through an ethanol/dry ice water trap and a thin, gas permeable silicone membrane (Cousins et al., 2006a, 2006b; Griffiths et al., 2007).
Stomatal Responses
To characterize the stomatal response of A. edulis plants, gas-exchange measurements were made with the LI-6400 portable gas-exchange system on two different sets of plants grown under identical conditions, young plants with an average of 10 leaves/plant and older plants with 20 to 25 leaves/plant. Data from the two sets of plants were combined in the final figures and tables. Light was provided by a red/blue LED light source (LI-6400-02B, LI-COR). Plants were dark adapted overnight at ambient CO2 conditions and then the uppermost expanded leaf was equilibrated in the gas-exchange leaf chamber at 364 μbar in the dark for 20 min. The blue/red light source was then turned on to give an irradiance of 2,000 μmol m−2 s−1 and maintained at that level for the rest of the experiment. After the initial 90 min in the light the CO2 concentration in the leaf chamber was decreased to 48 μbar and maintained for a further 90 min. Leaf chamber humidity and temperature were kept at 18 to 20 mmol mol−1 and 25°C, respectively, for the duration of the measurements. Flow rate over the leaf was 500 μmol s−1.
Steady stomatal conductance measurements were also made under the elevated CO2 growth conditions as described above by bringing the gas-exchange system into the growth chamber. Leaves were clamped into the leaf chamber and the growth chamber air (9.8 mbar of CO2) was allowed to flow over a leaf illuminated with 400 μmol quanta m−2 s−1 at a leaf temperature of 30°C. The leaf chamber humidity was not controlled but was similar between the wild-type and pp mutant 29.6 + 0.6 and 32.4 + 0.4 mmol mol−1, respectively. Plants were subsequently transferred from the elevated CO2 growth cabinets to air and a leaf was immediately placed into a gas-exchange chamber under the same measurement conditions except the CO2 concentration was 360 μbar and the leaf chamber humidity was controlled at 30.01 + 0.01 for both the wild-type and pp mutant (Fig. 3).
Determination of Stomatal Numbers
Stomatal numbers were determined from the same or similar leaves as used for gas-exchange measurements, from silicone rubber impressions taken from both sides of the leaves (von Caemmerer et al., 2004a). Stomata and epidermal cells were counted from positives made from the impressions with nail polish, in 10 different fields of view per leaf, with a compound microscope using a magnification of 200-fold. Digital photographs of each field were taken and cells counted with the publicly available Image J software (http://rsb.info.nih.gov/ij/).
Calculations of 13CO2 Discrimination
To calculate the conductance to CO2 diffusion from intercellular airspace to the site of Rubisco carboxylation in the BSCs in the pp mutant we used the model of C3 carbon isotope discrimination (Δ13C3) developed by Farquhar et al. (1982). This model is given as:
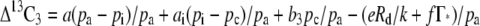 |
(1) |
where pa, pi, and pc represent the pCO2 of the air surrounding the leaf, in the intercellular air spaces and at the site of Rubisco carboxylation, respectively; a (4.4‰) is the fractionation during diffusion of CO2 in air, ai is the combined fractionation due to dissolution and diffusion of CO2 in water (1.8‰), and the fractionation by Rubisco is b3 = 30‰ (Roeske and Oleary, 1984). Γ* is the CO2 compensation point in the absence of day respiration. Rd is the rate of mitochondrial respiration, e and f are the discrimination factors of respiration and photorespiration with respect to the average carbon composition associated with respiration and photorespiration, respectively, and k is the Rubisco carboxylation efficiency (Farquhar et al., 1982; Evans et al., 1986). The carboxylation efficiency is given by
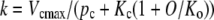 |
(2) |
where Vcmax denotes the maximal Rubisco activity, Kc and Ko are the Michaelis Menten constants for CO2 and O2, respectively, and O stands for O2 partial pressure.
The discrimination that would occur if the partial pressure of CO2 in the chloroplast equals the intercellular pCO2 and ignoring fractionations associated with respiration and photorespiration is usually given by
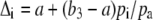 |
(3) |
Subtracting Equation 3 from Equation 1 shows that the difference between the Δi and the measured Δ13C3 is inversely proportional to the conductance to CO2 diffusion from the intercellular airspace to the site of Rubisco carboxylation (gi; Evans et al., 1986; von Caemmerer and Evans, 1991; Evans and von Caemmerer, 1996):
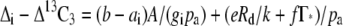 |
(4) |
such that gi can be estimated after rearranging Equation 4 from
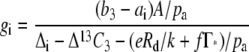 |
(5) |
The model of C4 carbon isotope discrimination (Δ13C4) from Farquhar (1983) was used to determine which factors in the model would influence Δ13C4 consistent with our experimental data in the Pp mutant and wild-type plants. The simplified model predicts that
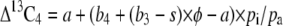 |
(6) |
where s (1.8‰) is the fractionation during CO2 leakage from the BSCs. The combined fractionation of PEPC and the isotopic equilibrium during dissolution of CO2 and conversion to bicarbonate (b4) was calculated as (Farquhar, 1983)
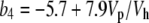 |
(7) |
indicating that the fractionation when CO2 and 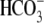 are not at equilibrium depends on the rate of CO2 hydration (Vh) and the rate of PEP carboxylation (Vp).
are not at equilibrium depends on the rate of CO2 hydration (Vh) and the rate of PEP carboxylation (Vp).
Calculations of C18OO Isotope Discrimination
Discrimination against C18OO (Δ18O) when water and CO2 at the site of exchange are at full isotopic equilibrium (θ = 1) can be predicted as (Farquhar and Lloyd, 1993)
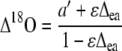 |
(8) |
where a′ is the diffusional discrimination (7.7‰) and ɛ is calculated as pm/(pa − pm) (Fig. 3, solid line). The 18O enrichment of CO2 compared to the atmosphere at the site of exchange in full oxygen isotope equilibrium with the water was calculated as
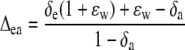 |
(9) |
(Cernusak et al., 2004). The equilibrium fractionation between water and CO2 (ɛw) was 40.17‰ at 30°C (Cernusak et al., 2004).
The δ18O of water at the sites of evaporation within a leaf (δe) can be estimated from the Craig and Gordon model of evaporative enrichment (Craig and Gordon, 1965; Farquhar and Lloyd, 1993)
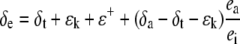 |
(10) |
where ea and ei are the vapor pressures in the atmosphere and the leaf intercellular spaces. δa and δt are the isotopic composition of water vapor in the air and transpired by the leaf, respectively. The kinetic fractionation during diffusion of water from leaf intercellular air spaces to the atmosphere (ɛk) and the equilibrium fractionation between liquid water and water vapor (ɛ+) was calculated according to Cernusak et al. (2004) and Cousins et al. (2006b). During the online gas-exchange measurements we let the leaves remain in steady-state conditions for a minimum of 1.5 h and under those conditions the value of δt is equal to the isotopic composition of source water, the water taken up by the plant (δs = −5.3 ± 0.3; Harwood et al., 1998).
The proportion of CO2 in isotopic equilibrium with water at the site of oxygen exchange (θ) can be estimated from
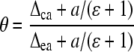 |
(11) |
where Δca is the oxygen isotope composition of CO2 at the site of exchange during photosynthesis (Gillon and Yakir, 2000a; Cousins et al., 2006b).
It has been suggested that the extent of θ in a leaf can also be calculated from in vitro CA assays coupled with the unidirectional flux of CO2 into the leaf (Gillon and Yakir, 2000a, 2000b, 2001) from the equation initially developed by Mills and Urey (1940):
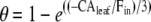 |
(12) |
where CAleaf/Fin represents the mean number of hydration reactions for each CO2 molecule inside the leaf (Gillon and Yakir, 2001). Leaf CA activity (CAleaf) is determined as the product of the CA hydration rate constant (kCA, mol m−2 s−1 bar−1) and the mesophyll pCO2 (pm). The rate constant kCA is calculated from in vitro measurements of CA activity in leaf extracts (see below). The gross influx of CO2 into a leaf (Fin = gt pa; where gt is the total conductance of CO2 from the atmosphere to the site of CO2-water oxygen exchange; Gillon and Yakir, 2000a), as well as pm determine the residence time (τ = pm/Fin) of CO2 within the leaf. The relationship of CAleaf/Fin indicates that conditions that influence pa, pm, gt, or kCA can alter the value of θ.
Enzyme Activities
Enzyme activities were determined on approximately 1 cm2 discs taken from the same leaves used for gas exchange. Leaf samples were collected after the gas-exchange measurements and subsequently frozen in liquid nitrogen and stored at −80°C. Tissue was ground on ice in 600 μL of extraction buffer (50 mm HEPES-KOH, pH 7.4, 10 mm dithiothreitol, 1% polyvinylpolypyrrolidone, 1 mm EDTA, and 0.1% Triton) with 20 μL of protease inhibitor cocktail (Sigma) and briefly centrifuged. PEPC activity was determined by placing 20 μL of leaf extract in 1 mL of assay buffer (100 mm EPPS-NaOH pH 8.0, 20 mm MgCl2, 1 mm EDTA, 0.2 mm NADH, 5 mm Glc-6-P, 1 mm NaHCO3, and 12 units of malate dehydrogenase). The reaction was initiated with 4 mm PEP and the rate of NADH consumption was determined by the absorbance change at 340 nm.
CA activity was measured on leaf extracts using mass spectrometry to measure the rates of 18O2 exchange from labeled 13C18O2 to 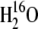 (Badger and Price, 1989; von Caemmerer et al., 2004b; Cousins et al., 2006a, 2006b). Measurements of leaf extracts were made at 25°C with a subsaturating total carbon concentration of 1 mm. The hydration rates were calculated from the enhancement in the rate of 18O loss over the uncatalyzed rate and the nonenzymatic first order rate constant was applied (pH 7.4, appropriate for the mesophyll cytosol). The CA activity was reported as a first order rate constant kCA (mol m−2 s−1 bar−1) and kCApm gives the in vivo CA activity at that particular cytosolic pCO2.
(Badger and Price, 1989; von Caemmerer et al., 2004b; Cousins et al., 2006a, 2006b). Measurements of leaf extracts were made at 25°C with a subsaturating total carbon concentration of 1 mm. The hydration rates were calculated from the enhancement in the rate of 18O loss over the uncatalyzed rate and the nonenzymatic first order rate constant was applied (pH 7.4, appropriate for the mesophyll cytosol). The CA activity was reported as a first order rate constant kCA (mol m−2 s−1 bar−1) and kCApm gives the in vivo CA activity at that particular cytosolic pCO2.
Epidermal Preparations
A fraction enriched in epidermal tissue was prepared by adapting the method of Kopka et al. (1997). A young expanding leaf was picked, the major veins were removed and discarded, and the rest was blended with 250 mL of chilled distilled water with a Sorvall Omni Mixer blender at maximum speed, with four pulses of 30 s each and waiting 30 s between pulses. The resulting epidermal fragments were rinsed with 300 mL of chilled distilled water on a 100 to 149 μm Nytal mesh to rid them of contaminating mesophyll cells. The epidermal fragments were drained of excess water, disrupted by grinding with mortar and pestle in liquid nitrogen for 3 min, and stored at −80°C until later use. The resulting fraction was highly enriched in epidermis compared to mesophyll cells (less than 1 mesophyll cell per mm2 epidermal fragment was observed under the compound microscope). Different blending regimes and filtration methods were ineffective in decreasing the contamination of epidermal fragments with bundle sheaths, so that the final preparations routinely contained a 10% to 20% contamination with bundle sheaths and vascular tissue as determined visually with the light microscope.
Protein Extraction and Immunoblotting
Soluble proteins from 1.28 cm2 leaf discs or 100-mg of epidermal fragments were extracted on ice in 0.7 mL of extraction buffer containing 50 mm HEPES-KOH, pH 7.8, 5 mm MgCl2, 2 mm EDTA, 5 mm dithiothreitol, 1% (w/v) polyvinylpolypyrrolidone, 0.1% (v/v) Triton X-10, and 4% (v/v) of protease inhibitor cocktail, using a 2-mL glass homogenizer. Samples were centrifuged in a cooled microcentrifuge at maximum speed for 4 min. The green pellet was discarded and the supernatant was brought to a final concentration of SDS of 2% (w/v) and heated to 65°C in a water bath for 10 min.
Protein concentration in the samples was determined with the bicinchoninic acid method (BCA Protein Assay kit, Pierce) prior to addition of SDS. Samples were prepared for gel loading by adding 0.25 volumes of Bio-Rad XT sample buffer (Bio-Rad). Thirty micrograms of total protein were loaded per gel well. Proteins were separated by electrophoresis on NuPAGE Bis-Tris precast gels (4%–12% acrylamide concentration, Novex) using the manufacturer-specified buffer system and blotted onto nitrocellulose membranes. Blots were probed with polyclonal antibodies raised against tobacco (Nicotiana tabacum) Rubisco and recombinant maize (Zea mays) PEPC, and with anti-Ig G alkaline phosphatase conjugate (Bio-Rad) as secondary antibody. Blots were developed using the AttoPhos fluorescence substrate system (Promega). Epidermal PEPC in the pp mutant was compared to wild type using Image Quant (Molecular Dynamics) to determine the relative abundance of the PEPC protein labeled by immunoblot from extractions prepared from three individual wild-type and four individual pp mutants.
Statistical Analysis
An ANOVA was conducted and Student's t test in STATISTICA (version 6.0 StatSoft). Tukey's honestly significant difference tests were used for post hoc comparisons.
Acknowledgments
We thank Dr. Spencer Whitney for the Rubisco and Dr. Tsuyoshi Furumoto for PEPC antibodies. We are thankful to Dr. Louisa Dever for the original isolation of the LaC4 2.16 PEPC-deficient mutant of A. edulis and Jessica Janek for her technical assistance.
This work was supported in part by a National Science Foundation international postdoctoral fellowship (to A.B.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter J. Lea (p.lea@lancaster.ac.uk).
Open Access articles can be viewed online without a subscription.
References
- Allaway WG (1973) Accumulation of malate in guard cells of Vicia faba during stomatal opening. Planta 110 63–70 [DOI] [PubMed] [Google Scholar]
- Andreo CS, Gonzales D, Inlesias A (1987) Higher plant phosphoenolpyruvate carboxylase: structure and regulation. FEBS Lett 213 1–8 [Google Scholar]
- Asai N, Nakajima N, Tamaoki M, Kamada H, Kondo N (2000) Role of malate synthesis mediated by phosphoenolpyruvate carboxylase in guard cells in the regulation of stomatal movement. Plant Cell Physiol 41 10–15 [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD (1989) Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol 89 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ (2005) Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new news on old paradigms. Plant Cell Environ 28 1396–1409 [Google Scholar]
- Cernusak LA, Farquhar GD, Wong SC, Stuart-Williams H (2004) Measurement and interpretation of the oxygen isotope composition of carbon dioxide respired by leaves in the dark. Plant Physiol 136 3350–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, Oleary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47 273–298 [DOI] [PubMed] [Google Scholar]
- Cotelle V, Pierre JN, Vavasseur A (1999) Potential strong regulation of guard cell phosphoenolpyruvate carboxylase through phosphorylation. J Exp Bot 50 777–783 [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S (2006. a) Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis: insights from antisense RNA in Flaveria bidentis. Plant Physiol 141 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S (2006. b) A transgenic approach to understanding the influence of carbonic anhydrase on C18OO discrimination during C4 photosynthesis. Plant Physiol 142 662–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig H, Gordon LI (1965) Deutrium and oxygen-18 variations in the ocean and the marine atmosphere. In E Tongiorgi, ed, Proceedings of a Conference on Stable Isotopes in Oceanographic Studies and Paleotemperatures. Consiglio Nazionale delle Ricerche, Laboratorie Geologia Nuclear, Pisa, Italy, pp 9–130
- Dever LV, Bailey KJ, Lacuesta M, Leegood RC, Lea PJ (1996) The isolation and characterization of mutants of the C4 plant Amaranthus edulis. C R Acad Sci Ser III Sci Vie 319 951–959 [Google Scholar]
- Dever LV, Bailey KJ, Leegood RC, Lea PJ (1997) Control of photosynthesis in Amaranthus edulis mutants with reduced amounts of PEP carboxylase. Aust J Plant Physiol 24 469–476 [Google Scholar]
- Dever LV, Blackwell RD, Fullwood NJ, Lacuesta M, Leegood RC, Onek LA, Pearson M, Lea PJ (1995) The isolation and characterization of mutants of the C4 photosynthetic pathway. J Exp Bot 46 1363–1376 [Google Scholar]
- Dever LV, Pearson M, Ireland RJ, Leegood RC, Lea PJ (1998) The isolation and characterization of a mutant of the C4 plant Amaranthus edulis deficient in NAD-malic enzyme activity. Planta 206 649–656 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher-plants. Aust J Plant Physiol 13 281–292 [Google Scholar]
- Evans JR, vonCaemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD (1983) On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol 10 205–226 [Google Scholar]
- Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In GD Farquhar, ed, Stable Isotopes and Plant Carbon-Water Relations. Academic Press, New York, pp 47–70
- Farquhar GD, Oleary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust J Plant Physiol 9 121–137 [Google Scholar]
- Flanagan LB, Ehleringer JR (1998) Ecosystem-atmosphere CO2 exchange: interpreting signals of change using stable isotope ratios. Trends Ecol Evol 13 10–14 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Hatch MD, Jenkins CLD (2000) C4 photosynthesis: mechanism and regulation. In S von Caemmerer, ed, Photosynthesis: Physiology and Metabolism, Vol 9. Academic Press, San Diego, pp 435–457
- Gehlen J, Panstruga R, Smets H, Merkelbach S, Kleines M, Porsch P, Fladung M, Becker I, Rademacher T, Hausler RE, et al (1996) Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum. Plant Mol Biol 32 831–848 [DOI] [PubMed] [Google Scholar]
- Ghashghaie J, Badeck FW, Lanigan G, Nogues S, Tcherkez G, Deleens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem Rev 2 145–161 [Google Scholar]
- Gillon JS, Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ 20 1217–1230 [Google Scholar]
- Gillon JS, Yakir D (2000. a) Internal conductance to CO2 diffusion and (COO)-O18 discrimination in C3 leaves. Plant Physiol 123 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon JS, Yakir D (2000. b) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against (COO)-O18 during photosynthesis. Plant Cell Environ 23 903–915 [Google Scholar]
- Gillon JS, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the O18 content of atmospheric CO2. Science 291 2584–2587 [DOI] [PubMed] [Google Scholar]
- Griffiths H, Cousins AB, Badger MR, von Caemmerer S (2007) Discrimination in the dark: resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase activity during the crassulacean acid metabolism cycle. Plant Physiol 143 1055–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood KG, Gillon JS, Griffiths H, Broadmeadow MSJ (1998) Diurnal variation of delta(CO2)-C-13, delta(COO)-O-18-O-16 and evaporative site enrichment of delta(H2O)-O-18 in Piper aduncum under field conditions in Trinidad. Plant Cell Environ 21 269–283 [Google Scholar]
- Hatch MD (1987) C4 photosynthesis—a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895 81–106 [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD (1992) Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19 263–285 [Google Scholar]
- Igamberdiev AU, Mikkelsen TN, Ambus P, Bauwe H, Lea PJ, Gardestrom P (2004) Photorespiration contributes to stomatal regulation and carbon isotope fractionation: a study with barley, potato and Arabidopsis plants deficient in glycine decarboxylase. Photosynth Res 81 139–152 [Google Scholar]
- Izui K, Matsumura H, Furumoto T, Kai Y (2004) Phosphoenolpyruvate carboxylase: a new era of structural biology. Annu Rev Plant Biol 55 69–84 [DOI] [PubMed] [Google Scholar]
- Kanai R, Edwards GE (1999) The biochemistry of C4 photosynthesis. In R Monson, ed, C4 Plant Biology. Academic Press, San Diego, pp 49–87
- Kiirats O, Lea PJ, Franceschi VR, Edwards GE (2002) Bundle sheath diffusive resistance to CO2 and effectiveness of C4 photosynthesis and refixation of photorespired CO2 in a C4 cycle mutant and wild-type Amaranthus edulis. Plant Physiol 130 964–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka J, Provart NJ, Müller Röber B (1997) Potato guard cells respond to drying soil by a complex change in the expression of genes related to carbon metabolism and turgor regulation. Plant J 11 871–882 [DOI] [PubMed] [Google Scholar]
- Ku MSB, Edwards GE (1975) Photosynthesis in mesophyll protoplasts and bundle sheath cells of various types of C4 plants. V. Enzymes of respiratory metabolism and energy utilizing enzymes of photosynthetic pathways. Z Pflanzenphysiol 77 16–32 [Google Scholar]
- Kubásek J, Šetlík J, Dwyer S, Šantruc J (2007) Light and growth temperature alter carbon isotope discrimination and estimated bundle sheath leakiness in C4 grasses and dicots. Photosynth Res 91 47–58 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Thomas M, Vidal J (2003) From enzyme to plant biotechnology: 30 years of research on phosphoenolpyruvate carboxylase. Plant Physiol Biochem 47 533–539 [Google Scholar]
- Lepiniec L, Vidal J, Chollet R, Gadal P, Cretin C (1994) Phosphoenolpyruvate carboxylase: structure, regulation and evolution. Plant Sci 99 111–124 [Google Scholar]
- Maroco JP, Ku MSB, Edwards GE (1997) Oxygen sensitivity of C4 photosynthesis: evidence from gas exchange and chlorophyll fluorescence analyses with different C4 subtypes. Plant Cell Environ 20 1525–1533 [Google Scholar]
- Maroco JP, Ku MSB, Edwards GE (2000) Utilization of O2 in the metabolic optimization of C4 photosynthesis. Plant Cell Environ 23 115–121 [Google Scholar]
- Maroco JP, Ku MSB, Furbank RT, Lea PJ, Leegood RC, Edwards GE (1998. a) CO2 and O2 dependence of PS II activity in C4 plants having genetically produced deficiencies in the C3 or C4 cycle. Photosynth Res 58 91–101 [Google Scholar]
- Maroco JP, Ku MSB, Lea PJ, Dever LV, Leegood RC, Furbank RT, Edwards GE (1998. b) Oxygen requirement and inhibition of C4 photosynthesis—an analysis of C4 plants deficient in the C3 and C4 cycles. Plant Physiol 116 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G, Urey H (1940) The kinetics of isotopic exchange between carbon dioxide, bicarbonate ion, carbonate ion and water. J Am Chem Soc 62 1019–1026 [Google Scholar]
- Outlaw WH Jr, Lowry OH (1977) Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci USA 74 4434–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathi K, Raghavendra AS (1997) Both rubisco and phosphoenolpyruvate carboxylase are beneficial for stomatal function in epidermal strips of Commelina benghalensis. Plant Sci 124 153–157 [Google Scholar]
- Pearson CJ (1973) Daily changes in stomatal aperture and in carbohydrates and malate within epidermis and mesophyll of leaves of Commelina cyanea and Vicia faba. Aust J Biol Sci 26 1035–1044 [Google Scholar]
- Poincelot RP (1972) Intercelluar distribution of carbonic anhydrase in spinach leaves. Biochim Biophys Acta 258 637–642 [DOI] [PubMed] [Google Scholar]
- Roeske CA, Oleary MH (1984) Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23 6275–6284 [DOI] [PubMed] [Google Scholar]
- Schnabl H, Raschke K (1980) Potassium chloride as stomatal osmoticum in Allium cepa L., a species devoid of starch in guard cells. Plant Physiol 65 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kirk CA, Raschke K (1978) Presence of chloride reduces malate production in epidermis during stomatal opening. Plant Physiol 61 361–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2005) Guard cell metabolism and CO2 sensing. New Phytol 165 665–682 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia
- von Caemmerer S (2003) C4 photosynthesis in a single C3 cell is theoretically inefficient but may ameliorate internal CO2 diffusion limitations of C3 leaves. Plant Cell Environ 26 1191–1197 [Google Scholar]
- von Caemmerer S, Evans JR (1991) Determination of the average partial pressure of CO2 in chloroplast from leaves of several C3 plants. Aust J Plant Physiol 18 287–305 [Google Scholar]
- von Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77 191–207 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Hendrickson L, Quinn V, Vella N, Millgate AG, Furbank RT (2005) Reductions of Rubisco activase by antisense RNA in the C4 plant Flaveria bidentis reduces Rubisco carbamylation and leaf photosynthesis. Plant Physiol 137 747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004. a) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55 1157–1166 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price D, Badger MR, Furbank RT (1997. a) Carbon isotope discrimination during C4 photosynthesis: insights from transgenic plants. Aust J Plant Physiol 24 487–494 [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT (1997. b) Reduction of Ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiol 113 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Quinn V, Hancock NC, Price GD, Furbank RT, Ludwig M (2004. b) Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant Cell Environ 27 697–703 [Google Scholar]
- Wheeler RM, Mackowiak CL, Yorio NC, Sager JC (1999) Effects of CO2 on stomatal conductance: do stomata open at very high CO2 concentrations? Ann Bot (Lond) 83 243–251 [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker M (1996) Stomata, Ed 2. Chapman & Hall, London
- Wong SC, Cowan IR, Farquhar GD (1985) Leaf conductance in relation to rate of CO2 assimilation.1. Influence of nitrogen nutrition, phosphorus-nutrition, photon flux-density, and ambient partial-pressure of CO2 during ontogeny. Plant Physiol 78 821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir D, Sternberg Ld L (2000) The use of stable isotopes to study ecosystem gas exchange. Oecologia 123 297–311 [DOI] [PubMed] [Google Scholar]