Abstract
Ultra-performance liquid chromatography-tandem mass spectrometry was used to establish the cytokinin profile of the bryophyte Physcomitrella patens (Hedw.) B.S.G.; of 40 analyzed cytokinins, 20 were detected. cis-Zeatin-riboside-O-glucoside, N6-(Δ2-isopentenyl)adenosine-5′-monophosphate (iPRMP), and trans-zeatin-riboside-O-glucoside were the most abundant intracellular cytokinins. In addition, the aromatic cytokinins N6-benzyladenosine (BAR), N6-benzyladenine, meta-, and ortho-topolin were detected. Unexpectedly, the most abundant extracellular cytokinin was the nucleotide iPRMP, and its identity was confirmed by quadrupole time-of-flight mass spectrometry. The effects of overexpressing a heterologous cytokinin oxidase/dehydrogenase (CKX; EC 1.4.3.18/1.5.99.12) gene (AtCKX2 from Arabidopsis [Arabidopsis thaliana]) on the intracellular and extracellular distribution of cytokinins was assessed. In cultures of CKX-transformed plants, ultra-performance liquid chromatography-tandem mass spectrometry measurements showed that there were pronounced reductions in the extracellular concentrations of N6-(Δ2-isopentenyl)adenine (iP) and N6-(Δ2-isopentenyl)adenosine (iPR), but their intracellular cytokinin concentrations were only slightly affected. In vitro and in vivo measured CKX activity was shown to be strongly increased in the transformants. Major phenotypic changes observed in the CKX-overexpressing plants included reduced and retarded budding, absence of sexual reproduction, and abnormal protonema cells. In bud-induction bioassays with wild-type Physcomitrella, the nucleotides iPRMP, trans-zeatin-riboside-5′-monophosphate, BAR monophosphate, and the cis-zeatin forms cZ and cZR had no detectable effects, while the activities displayed by other selected cytokinins were in the following order: iP > tZ > N6-benzyladenine > BAR > iPR > tZR > meta-topolin > dihydrozeatin > ortho-topolin. The results on wild type and CKX transgenics suggest that extracellular iP and iPR are the main cytokinins responsible for inducing buds in the bryophyte Physcomitrella. Cytokinin profile is discussed regarding the evolution of cytokinin biosynthetic pathways.
Cytokinins play important roles as growth-regulating compounds in plants (Kieber, 2002). External applications of cytokinins to mosses have been shown to induce bud formation and, thus, the transition from filamentous, protonemic growth to the formation of gametophores (Bopp and Brandes, 1964; Reski and Abel, 1985). However, knowledge of the endogenous cytokinin profiles of mosses is incomplete, and it is unclear how their intracellular and extracellular distributions regulate developmental processes. We have therefore attempted to establish the cytokinin profile of Physcomitrella patens, a model organism for plant development and metabolism studies (Cove et al., 2006).
Naturally occurring cytokinins are N6-substituted adenine derivatives bearing either an isoprenoid or an aromatic side chain. Isoprenoid forms include N6-(Δ2-isopentenyl)adenine (iP)- and zeatin (Z)-type cytokinins, which are characterized by the side-chain hydroxylation. Possible modifications of N6-isoprenoid side chains are hydroxylation of iP- to Z-type cytokinins, cis-trans isomerization of the hydroxyl group by cis-trans-Z isomerase, formation of Z-O-glucosides or Z-O-xylosides, reduction of Z to dihydrozeatin (DHZ), and the complete cleavage of the side chain by cytokinin oxidase/dehydrogenase (CKX; EC 1.4.3.18/1.5.99.12). All of these N6 side-chain modifications can have pronounced effects on the hormonal activity of the compounds (for review, see Mok and Mok, 2001; Sakakibara, 2006).
Cytokinin bases and their corresponding ribosides and nucleotides can be interconverted, usually in reactions catalyzed by purine metabolizing enzymes (for review, see Chen, 1997), although a cytokinin-specific phosphoribohydrolase has been described recently, which converts cytokinin nucleotides directly to bases (Kurakawa et al., 2007). In most bioassays cytokinin bases are reported to be more active than the corresponding ribosides. The biological activity of cytokinin nucleotides is still unclear, since they usually show no activity in bioassays but can bind to certain cytokinin receptors (Spichal et al., 2004).
The adenine moiety of cytokinins can also be glycosylated, resulting in the formation of either N7- or N9-glucosides, which are much less active than the unglycosylated forms (Letham et al., 1983).
Two cytokinin biosynthesis pathways are known to be present in plants. The direct de novo synthesis of free cytokinins is catalyzed by adenylate isopentenyltransferases (IPTs), which preferentially alkylate ADP and ATP to the corresponding cytokinin nucleotides (Kakimoto, 2001). The second, indirect pathway involves isopentenylation of tRNAs that recognize UNN codons. These tRNAs are known to be modified at the A37 position by the activity of tRNA-IPTs (Taller, 1994). The turnover of the UNN-recognizing tRNAs liberates cytokinin nucleotides. Miyawaki et al. (2006) have recently shown that adenylate IPTs are responsible for the bulk of iP- and trans-zeatin (tZ)-type cytokinin synthesis in Arabidopsis (Arabidopsis thaliana), while tRNA-IPTs preferentially generate cis-zeatin (cZ)-type cytokinins.
CKX activity was first demonstrated by Paces et al. (1971), who measured the degradation of radiolabeled N6-(Δ2-isopentenyl)adenosine (iPR) to adenosine in crude extracts prepared from tobacco (Nicotiana tabacum) cell cultures. Following publication of a study by Whitty and Hall (1974), it was generally assumed that molecular oxygen was essential for CKX activity, which led to its designation as a cytokinin oxidase. However, it was subsequently shown that CKX can use a variety of electron acceptors in the absence of molecular oxygen and that the enzyme can act as a dehydrogenase (Galuszka et al., 2001; Frebort et al., 2002). The first CKX gene, from maize (Zea mays), was concurrently cloned by Morris et al. (1999) and Houba-Hérin et al. (1999). Transgenic experiments in which CKX has been overexpressed, thus generating cytokinin-deficient plants, have provided new insights into the function of cytokinins in tobacco and Arabidopsis (Werner et al., 2001, 2003b, 2006).
Overexpression of Arabidopsis AtCKX1 or AtCKX2 has contrasting effects in the shoots and roots of tobacco plants. In experiments reported by Werner et al. (2001), the shoots showed stunted growth with smaller apical meristems and dramatically reduced numbers of leaf cells, while the root meristems were larger and consequently the root systems grew more rapidly and branched more profusely than in the corresponding wild type. In addition, analyses with AtCKX-green fluorescence fusion proteins have revealed that the subcellular locations of specific CKX proteins differs (Werner et al., 2003b), and AtCKX2 was shown to be secreted into the apoplastic compartment.
As AtCKX2 expressed in transgenic tobacco, compared to other AtCKX isoforms, was shown to highly increase the degradation of iP-type cytokinins (Galuszka et al., 2007), which were considered to be major cytokinins in Physcomitrella (Wang et al., 1980; von Schwartzenberg, 2006), this gene was selected for heterologous overexpression in moss.
The moss Physcomitrella represents an example of an evolutionarily primitive land plant. As mosses generally are regarded as living fossils, the analysis of their cytokinin physiology sheds light on the evolution of cytokinin-mediated growth regulation. We present a comprehensive analysis of the intracellular and extracellular distributions of cytokinins in moss, together with data on their activity, and the influence of cytokinin deficiency on its developmental processes.
RESULTS
Generation of CKX-Overexpressing Plants
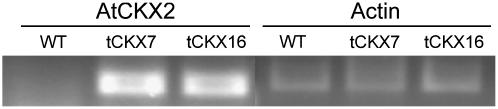
cDNA encoding the AtCKX2 gene from Arabidopsis was placed under the control of the rice (Oryza sativa) actin1 promoter to construct the expression vector, designated pHP_act1_AtCKX2. After polyethylene glycol (PEG)-mediated transformation of Physcomitrella protoplasts, transformants resistant to G418 were selected. From 30 lines the plants tCKX7 and tCKX16 were selected for further characterization at the molecular, metabolic, and phenotypic levels. The integration of the transgene into high Mr genomic DNA of Physcomitrella was confirmed by Southern blotting (data not shown), and the transcription of the transgene was shown by reverse transcription (RT)-PCR analysis, using specific primers for the AtCKX2 gene (Fig. 1).
Figure 1.
Results of an RT-PCR experiment demonstrating the expression of the AtCKX2 gene in the transgenic lines tCKX7 and tCKX16. The AtCKX2-specific primers amplify a 307-bp fragment. For the control gene PpACT3 (AY382283), a 320-bp fragment was amplified. Assays lacking reverse transcriptase displayed no amplification products, indicating the absence of interfering genomic DNA (not shown).
In Vitro CKX Activity
The CKX activities in wild-type, tCKX16, and tCKX7 plants were compared in radiometric assays using a copper-imidazole buffer (Table I). The CKX specific activity was up to approximately 27-fold higher in tissue extracts of the transformants than in corresponding wild-type extracts. Furthermore, the specific activity was up to 157-fold higher in protein preparations from the culture media of the transformants than in corresponding wild-type preparations, indicating that a considerable proportion of the recombinant CKX was secreted into the culture medium.
Table I.
Specific activity of CKX in cellular and extracellular protein preparations of 12-d-old liquid cultures (15°C) of wild-type P. patens and AtCKX2-overexpressing transformants (mean values ± sds)
| Specific Activity
|
||
|---|---|---|
| Tissue | Medium | |
| nmol adenine mg−1 protein h−1 | ||
| Wild type | 0.54 ± 0.014 | 0.06 ± 0.002 |
| tCKX7 | 11.09 ± 0.73 | 5.02 ± 0.69 |
| tCKX16 | 14.53 ± 0.64 | 9.42 ± 0.99 |
In Vivo Cytokinin Metabolism
To assess the effects of the transformation on cytokinin catabolism, the radiolabeled cytokinins N6-benzyl[2-3H]adenine ([2-3H]BA), N6-(Δ2-isopentenyl)[2-3H]adenine ([2-3H]iP), and N6-(Δ2-isopentenyl)[2-3H]adenosine ([2-3H]iPR) were added to the culture media of both the wild type and transformants.
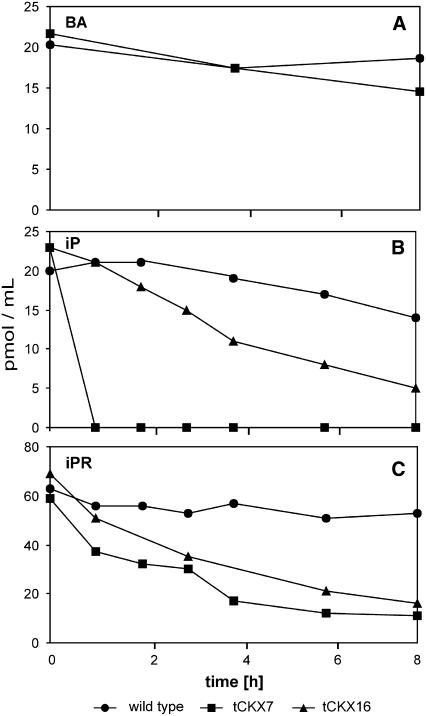
As a control, [2-3H]BA, which is a very poor substrate for CKX, was applied to wild-type and tCKX7 cultures at a concentration of 20 pmol/mL and its metabolism was monitored in the culture medium by HPLC coupled to online liquid scintillation counting. During 8 h of incubation, no significant differences in [2-3H]BA metabolization were found between the wild type and tCKX7 (Fig. 2A). However, when [2-3H]iP (20 pmol/mL), a preferred substrate of CKX, was applied, dramatic differences in its metabolization were found (Fig. 2B). The amount of external [2-3H]iP in the media of wild-type and tCKX16 cultures decreased within 8 h to 75% and 25% of initial levels, respectively, while in tCKX7 cultures the concentration fell extremely rapidly, to below the detection limit within an hour. Thus, tCKX7 and tCKX16 have greatly enhanced in vivo degradation capacity for [2-3H]iP. The degradation capacities of the wild type, tCKX16, and tCKX7 per unit mass of wet protonema (including the retained medium) were found to be 3, 10, and 91 pmol h−1 g−1, respectively.
Figure 2.
Time courses of extracellular concentrations of the radiolabeled cytokinins [2-3H]BA (A), [2-3H]iP (B), and [2-3H]iPR (C) in the culture medium during in vivo labeling of wild-type P. patens and the transformants tCKX16 und tCKX7. The substrate depletion was monitored by HPLC coupled to online liquid scintillation counting.
The riboside [2-3H]iPR was applied at a higher concentration (60 pmol/mL) to allow quantifiable amounts of labeled metabolites to be extracted from the tissues. Monitoring the external [2-3H]iPR concentration, again a stronger depletion was observed in the media of the transformant cultures (63%–77% decrease after 8 h of incubation) than in the wild-type culture medium (15% after 8 h; Fig. 2C).
To assess the effects of CKX overexpression with respect to the conversion products [2-3H]iP and [2-3H]iPR nucleotides, detailed analyses of culture media and tissue extracts were carried out.
In wild-type culture medium, the extracellular metabolite [2-3H]iP accumulated to concentrations of up to 15 pmol/mL within 20 h, while in the media of both of the transformants no [2-3H]iP was detectable after 20 h (data not shown), probably because any formed was rapidly degraded.
After 20 h of incubation with [2-3H]iPR, no radioactivity was found in the extracellular fractions of cytokinin nucleotides (data not shown).
The distribution of radioactivity among the extractable tissue-bound [2-3H]iPR metabolites is presented in Table II. Relatively small intracellular amounts of the [2-3H]iPR substrate (7.5%) remained after 20 h in wild-type tissues, while in the tCKX16 and tCKX7 transformants no detectable tissue-bound [2-3H]iPR remained after 20 and 4 h. Similarly, the main metabolite [2-3H]iP was detectable in wild-type tissues, but not in tCKX7 and tCKX16 tissues, after 20 h incubation.
Table II.
Distribution of extractable radioactivity in P. patens protonema tissue (150–242 mg FW) after feeding with [2-3H]iPR (60 pmol/mL) in a volume of 4 mL
Percentages related to total amount of extractable radioactivity are given in brackets. iPRDP, Isopentenyladenosine diphosphate; iPRTP, isopentenyladenosine triphosphate. d.l., Detection limit (approximately 0.01 pmol).
| Genotype | Time | iPR | iP | iP Nucleotides
|
Degradation Products | |
|---|---|---|---|---|---|---|
| iPRMP | iPRDP, iPRTP | |||||
| h | pmol/100 mg [%] | |||||
| Wild type | 4 | 0.1 [3.7] | 0.1 [4.0] | 0.5 [18.9] | 0.5 [20.0] | 1.4 [53.4] |
| 20 | 0.6 [7.5] | 1.3 [17.8] | 1.2 [15.6] | 0.7 [9.3] | 3.7 [49.8] | |
| tCKX7 | 4 | <d.l. | <d.l. | <d.l. | <d.l. | 14.9 [100.0] |
| 20 | <d.l. | <d.l. | <d.l. | 0.3 [2.9] | 11.2 [97.1] | |
| tCKX16 | 4 | 0.2 [3.0] | 0.3 [5.4] | 0.6 [10.5] | 0.3 [5.6] | 4.3 [75.5] |
| 20 | <d.l. | <d.l. | 0.2 [2.0] | 0.4 [3.6] | 10.0 [94.4] | |
The relative proportions of cytokinin nucleotides were also clearly reduced in the transformant cultures, and no [2-3H]iPRMP [N6-(Δ2-isopentenyl)adenosine-5′-monophosphate, iPRMP] was found in tCKX7 cultures. As a result of CKX overexpression, the metabolism of [2-3H]iPR was directed, as expected, toward the degradation products, which accounted for 75% to 100% of the extractable radioactivity in the transformants, but only for at most 53% in the wild type. In summary, the results of the labeling studies clearly demonstrate the functional overexpression of the heterologous CKX under in vivo conditions, revealing dramatic reductions in the levels of labeled cytokinin bases, ribosides, and nucleotides.
Native Cytokinins in Physcomitrella
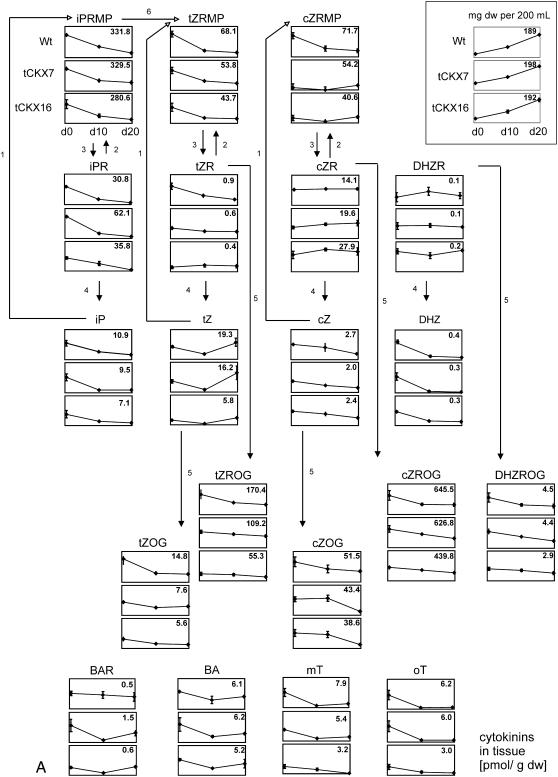
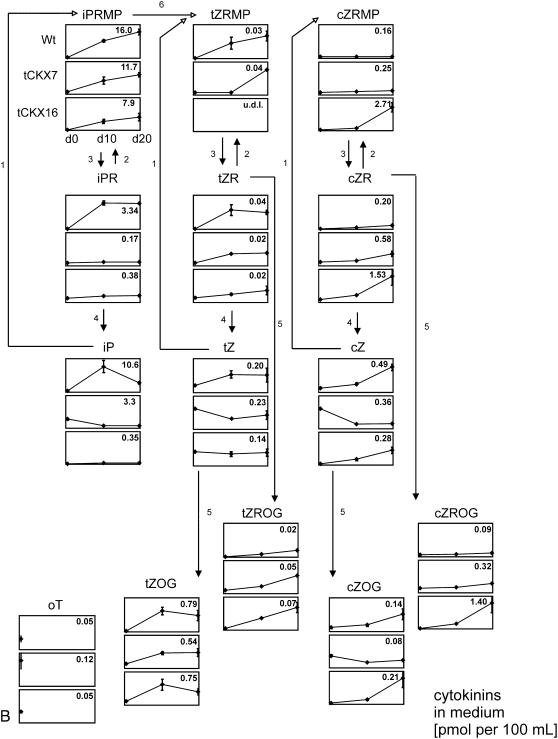
The contents of more than 40 isoprenoid and aromatic cytokinins in the tissues and culture media of the three investigated lines was monitored by sensitive ultra-performance liquid chromatography-electrospray tandem mass spectrometry (UPLC-MS/MS). Liquid cultures were washed with fresh medium at day 0 and harvested at three time points (days 0, 10, and 20) over a cultivation period of 20 d. Changes in the distribution of extracellular and intracellular cytokinins were monitored, thus allowing to estimate the amount of released cytokinins over the time course. All genotypes were grown and sampled in triplicate. Mean values and sds of the amounts of the cytokinins found in the tissues and media are shown in Figure 3, A and B, respectively, and the results are summarized in Table III and Supplemental Table S3 (see also Supplemental Tables S1 and S2).
Figure 3.
A, Concentrations of endogenously produced cytokinins in tissues of wild-type P. patens and CKX-overexpressing mutants (tCKX7 and tCKX16) at three time points (days 0, 10, and 20) as determined by UPLC-MS/MS. Three independent liquid cultures of each type were harvested and analyzed. Results are presented as mean values with sds. The y scale of the graph for each compound is identical, and in each graph the maximum cytokinin concentration is given in pmoles per gram DW at the top right. Growth curves are presented at the top (milligrams of tissue DW per 200 mL). Data are available as Supplemental Table S1. For abbreviations of cytokinins, see Supplemental List S1. 1, Adenine phosphoribosyltransferase; 2, adenosine kinase; 3, phosphatase/nucleotidase; 4, adenosine nucleosidase; 5, Z-O-glycosyltransferase; 6, cytochrome P450 monooxygenases. The postulated metabolic pathways (Schematic) are based on those presented by Sakakibara (2006). B, Concentrations of endogenously produced cytokinins in culture medium (pmoles per 100 mL) of wild-type P. patens and CKX-overexpressing mutants (tCKX7 and tCKX16) at three time points (days 0, 10, and 20). Some compounds that were found in the tissue extracts were not detectable in the culture medium (for further details, see A). Data are available as Supplemental Table S2. u.d.l., Under detection limit.
Table III.
Intracellular and extracellular distribution of groups of cytokinins calculated as pmoles produced per 200 mL of liquid culture
Data derived from analysis presented in Figure 3, A and B. u.d.l., Under detection limit.
| Tissue
|
Day | Medium
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| iP Type | tZ Type | cZ Type | DHZ Type | BAP Type | T Type | iP Type | tZ Type | cZ Type | DHZ Type | BAP Type | T Type | ||
| Wild type | 9.4 | 6.7 | 19.8 | 0.12 | 0.2 | 0.4 | 0 | 1.3 | 0.20 | 0.52 | u.d.l. | u.d.l. | 0.09 |
| 15.0 | 10.5 | 35.8 | 0.24 | 0.3 | 0.1 | 10 | 48.7 | 2.11 | 0.96 | u.d.l. | u.d.l. | u.d.l. | |
| 7.1 | 11.5 | 39.1 | 0.24 | 0.5 | 0.3 | 20 | 46.3 | 1.78 | 2.12 | u.d.l. | u.d.l. | u.d.l. | |
| tCKX7 | 14.0 | 6.3 | 25.7 | 0.17 | 0.3 | 0.4 | 0 | 7.0 | 0.58 | 1.09 | u.d.l. | u.d.l. | 0.23 |
| 16.6 | 10.0 | 53.2 | 0.32 | 0.2 | 0.2 | 10 | 16.3 | 1.28 | 0.76 | u.d.l. | u.d.l. | u.d.l. | |
| 13.9 | 8.9 | 35.2 | 0.01 | 0.4 | 0.3 | 20 | 24.0 | 1.58 | 2.43 | u.d.l. | u.d.l. | u.d.l. | |
| tCKX16 | 10.2 | 3.4 | 17.1 | 0.10 | 0.2 | 0.2 | 0 | 0.3 | 0.37 | 0.17 | u.d.l. | u.d.l. | 0.10 |
| 11.9 | 5.6 | 40.9 | 0.24 | 0.2 | 0.3 | 10 | 11.7 | 1.80 | 1.84 | u.d.l. | u.d.l. | u.d.l. | |
| 5.6 | 4.2 | 32.6 | 0.20 | 0.5 | 0.1 | 20 | 17.1 | 1.30 | 12.25 | u.d.l. | u.d.l. | u.d.l. | |
Cytokinin Profile in Wild-Type Tissue
The profiling revealed that all groups of isoprenoid cytokinins, such as iP-, tZ-, cZ-, and DHZ-type cytokinins, are present in Physcomitrella tissue, and that all of these groups were represented by detectable amounts of the bases, ribosides, and nucleotides, except DZRMP. In addition, O-glucosides of both the bases (tZOG and cZOG) and ribosides (cZROG and tZROG) of the cZ and tZ types of hydroxylated cytokinins were found (Fig. 3A; Table III; Supplemental Table S3).
The most abundant intracellular cytokinins were cZROG, followed by iPRMP, tZROG, cis-zeatin-riboside-5′-monophosphate (cZRMP), trans-zeatin-riboside-5′-monophosphate (tZRMP), cZOG, and iPR, for which maximum concentrations found were 646, 332, 170, 72, 68, 52, and 36 pmol/g dry weight (DW), respectively. All other detected cytokinins were present at concentrations ≤30 pmol/g DW.
Interestingly, the aromatic cytokinin bases BA, meta-topolin (mT), and ortho-topolin (oT) were also detected, but N6-benzyladenosine (BAR) was the only aromatic cytokinin riboside found, and no nucleotides of aromatic cytokinins were detectable.
Intracellular concentrations of most cytokinins (e.g. iPRMP) were highest at the beginning of the sampling period. However, concentrations of some metabolites (e.g. cZR and DZR) did not significantly change during cultivation, and levels of one (tZ) increased between days 10 and 20.
Most of the decrease in intracellular cytokinin contents was probably due to the release of cytokinins into the fresh culture medium in which the tissue was suspended at day 0 (Fig. 3A).
Influence of CKX Overexpression on Intracellular Cytokinins
Although strong effects of CKX overexpression on the exogenously applied tritiated cytokinins [2-3H]iPR and [2-3H]iP were observed in the short-term labeling experiments (see Fig. 2), it had a much less pronounced influence on endogenously produced cytokinins, and the intracellular content of cZR was even higher in the tCKX7 and tCKX16 transformants than in the wild type. The compound showing the most significant decrease was tZROG (especially in tCKX16), and concentrations of most of the other cytokinin metabolites showed only nonsignificant tendencies to be lower in the CKX overexpressors (Fig. 3A).
Profile of Cytokinins in Culture Medium
The UPLC-MS/MS analysis of the medium of wild-type cultures revealed that all groups of isoprenoid cytokinins detected in the tissue were also present in the wild-type medium. However, concentrations of the cytokinins cZRMP, DHZRMP, DHZR, and DHZ were below their respective detection limits (for abbreviations, see Supplemental List S1).
The concentrations of the extracellular cytokinins ranged between 0.08 and 16 pmol per 100 mL of medium. The major extracellular cytokinin was iPRMP (maximum, 16 pmol/100 mL in wild-type medium), followed by iP (10 pmol/100 mL) and iPR (3 pmol/100 mL). All other cytokinin forms were present at concentrations lower than 1 pmol/100 mL (Fig. 3B).
The identity of iPRMP in Physcomitrella medium was strongly supported using a combination of capillary liquid chromatography (CapLC module) and mass spectrometric analysis with a Q-Tof micro hybrid quadrupole time-of-flight mass spectrometer, enabling high resolution identification of cytokinin derivatives (Supplemental Fig. S1). Taken together, the corresponding fragmentation pattern and the exact mass confirmed the presence of iPRMP as a major cytokinin in Physcomitrella culture media.
The only aromatic cytokinin in the media was oT, detected in trace quantities at day 0; concentrations of all other aromatic cytokinins and oT at all other sampling times were below the detection limit.
In contrast to the intracellular fractions, the extracellular cytokinins mostly accumulated over time; the only deviations from this pattern were that concentrations of iP declined between days 10 and 20, and concentrations of cZRMP generally remained stable throughout the sampling period (Fig. 3B).
Influence of CKX Overexpression on Extracellular Cytokinins
The influence of CKX overexpression on extracellular cytokinin levels was most pronounced for the iP-type derivatives; iPR concentrations were approximately 19-fold lower in tCKX7 medium than in wild-type medium (0.17 versus 3.3 pmol per 100 mL), and iP concentrations were 55- and 30-fold reduced in tCKX7 and tCKX16, respectively (0.19 and 0.35 pmol per 100 mL in the tCKX7 and tCKX16 media, respectively, and 10.6 pmol per 100 mL in the wild-type medium, on day 10, when the concentrations were maximal; Fig. 3B).
In addition, extracellular concentrations of the cytokinin nucleotide iPRMP were reduced in both of the transformant cultures. Unexpectedly, however, extracellular concentrations of some cytokinins (including tZROG, cZROG, cZRMP, and cZR) were higher in cultures of the transformants than in wild-type cultures.
In summary, it can be concluded that CKX overexpression also affects levels of endogenously produced cytokinins. The strongest reductions were found for iP and iPR in the culture medium.
Consequences of Cytokinin Deficiency in Physcomitrella
Cytokinin-deficient transformants showed numerous phenotypical changes at various levels.
(1) Following protoplast transformation using the AtCKX2-carrying construct (pHP_act1_AtCKX2), far fewer stable transgenic lines were obtained than from protoplasts transformed with the control plasmid pHP23 (two and 20, respectively, from approximately 106 protoplasts). Clearly, therefore, expression of the AtCKX2 gene has a strongly negative influence on protoplast vitality and/or regeneration capacity.
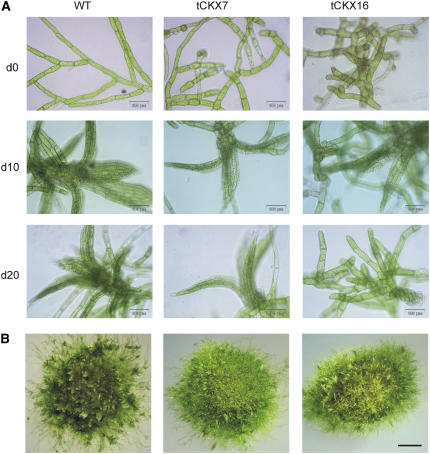
(2) At the protonema level, the morphology of the filaments was strongly altered in both the tCKX7 and tCKX16 transformants. The morphology of the chloronema of the transgenics was abnormal and irregular (Fig. 5A), mainly due the occurrence of a high number of cells that were substantially shorter (31–58 μm, versus ≥60 μm usually) but wider (diameter, up to 48 μm, versus <33 μm usually) than wild-type cells (Supplemental Fig. S2). However, although the material was clonal, not all protonema cells of the CKX transformants displayed altered cell morphology, and the size of a substantial proportion of the cells remained unaffected by cytokinin deficiency, with dimensions comparable to the wild type.
Figure 5.
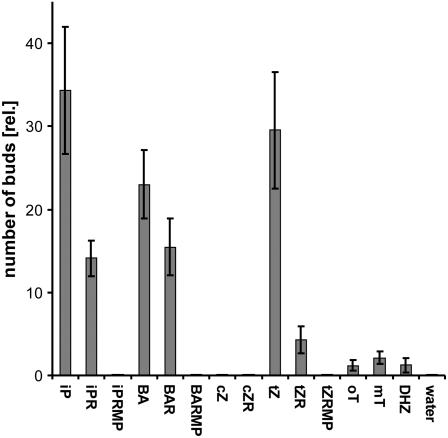
Activity of various cytokinins in the P. patens budding bioassay (wild type) on agar plates. All compounds were applied to medium at a concentration of 0.5 μmol/L. The number of induced buds was counted after 6 d (mean values calculated from buds counted in 20–40 microscopic fields, bars represent sds).
(3) Budding was impaired in untreated tCKX7 and tCKX16 protonemata growing in both liquid and agar culture, the time until buds appeared being longer and the numbers of buds produced lower in the transformant cultures than in the wild-type cultures (Fig. 4B).
Figure 4.
A, Micrograph of protonema and developing gametophores in liquid cultures of wild-type P. patens and the transformants tCKX7 and tCKX16. Pictures were taken on days 0, 10, and 20 (d0, d10, and d20) and correspond to cultures from which the data displayed in Figure 3, A and B, were obtained; bars represent 100 μm. B, Morphology of cultures grown on agar after 8 weeks; bar represents 1 mm.
(4) Although tCKX7 and tCKX16 transformants produced gametophores, no sexual reproduction was observed. Since the formation of neither archegonia nor antheridia was observed, the transition to the sporophytic generation seems to be blocked at the level of gametangia development (data not shown). In general, the development of gametophores was delayed and, moreover, they were often smaller than wild-type gametophores (Fig. 4A).
Since exogenously applied cytokinins are known to induce bud formation in moss protonema, we applied iP to assess whether the increased cytokinin degradation in the transformants influenced the budding response. The transformants formed significantly fewer buds than the wild type, even when iP was applied at concentrations of 500 nmol/L (Supplemental Fig. S3). The tCKX7 transformant displayed an especially weak budding response, concentrations of 50 nmol/L or more being required to induce any detectable response. Control assays with BA revealed comparable budding responses for the wild type and CKX transformants (data not shown).
Activity of Cytokinins in Physcomitrella Bioassays
The activities of selected representatives of the various types of cytokinins from the broad spectrum of 20 endogenous cytokinins detected in the UPLC-MS/MS analysis (Fig. 3, A and B) were assayed in a bud-induction bioassay with wild-type cultures. The most potent bud-inducing cytokinin was found to be iP, followed in order of decreasing activity by tZ, BA, BAR, iPR, and tZR. The cytokinins DHZ, mT, and oT exhibited very weak activities, while cZ, cZR, and the nucleotides iPRMP, tZRMP, and BAR monophosphate (BARMP) showed no activity at all (Fig. 5).
DISCUSSION
Since the discovery of cytokinins, their functions and roles in plant development have mainly been assessed by exogenously administering cytokinins or by creating IPT-transgenic plants with increased rates of cytokinin biosynthesis. However, since the identification of CKX genes (Houba-Hérin et al., 1999; Morris et al., 1999), new approaches for evaluating the role of cytokinins have become available, resulting from the possibility to experimentally decrease the endogenous cytokinin contents in CKX-overexpressing plants.
Here, we used the rice actin1 promoter (Wang et al., 1992) to constitutively overexpress the AtCKX2 gene and transformed moss protoplasts by PEG-mediated direct gene transfer. The regeneration of CKX-overexpressing plants proved to be extremely difficult, and we were only able to isolate two stable transformants, tCKX7 and tCKX16. Both lines were shown to express the transgene in RT-PCR studies (Fig. 1).
CKX Enzyme Activity in Vitro
In vitro enzyme assays (Table I) showed that CKX specific activity was up to 27-fold higher in the tissues of the transformants than in wild-type tissues, and up to 157-fold higher in concentrated protein preparations from their culture media. Similarly, significantly higher stimulation of CKX activity in the media than in the cells has been reported for Saccharomyces cerevisiae cultures expressing the AtCKX2 gene (Werner et al., 2001). The finding that most of the recombinant protein was targeted to the extracellular compartment is consistent with the fact that the coding sequence used for vector construction contained the AtCKX2 signal sequence, which (as for some other CKX proteins) reportedly directs the gene product to the secretory pathway (Schmülling et al., 2003).
Metabolites of Radiolabeled Cytokinins in Media
Cultures were fed with radiolabeled cytokinins to confirm that the capacity of the transformants to degrade cytokinins had been enhanced, as intended. In a control experiment, [2-3H]BA, which has been reported to be either a nonsubstrate (Armstrong, 1994) or a very poor substrate for CKX (Laloue and Fox, 1989; Galuszka et al., 2007), was applied. In accordance with these reports, only a slight decrease in extracellular [2-3H]BA was found in both wild-type and tCKX7 cultures during 8 h of incubation (Fig. 2A). No significant differences were observed between wild-type and tCKX cultures with respect to extracellular [2-3H]BA depletion. In contrast to [2-3H]BA, strong reductions in the extracellular concentration of the substrate cytokinins [2-3H]iP and [2-3H]iPR (Fig. 2, B and C) were observed. The absence of observable differences between the transformants in the depletion of [2-3H]iPR, such as seen in the labeling experiment with [2-3H]iP, may be due to their uptake capacity for [2-3H]iPR being close to saturation (Fig. 2, B and C).
Metabolites of Radiolabeled Cytokinins in Tissue Extracts
The increased cytokinin breakdown capacity of the CKX transformants was also confirmed by the analysis of tissue extracts. After 20 h of incubation, neither the applied substrate [2-3H]iPR nor its metabolic product [2-3H]iP were detectable in the transformants (Table II). This is in accordance with the higher amount of degradation products found in the transformants and indicates that their reductions in concentrations of cytokinin metabolites were indeed due to enhanced CKX activity. In the experiments with tCKX7 cultures, all of the extractable radioactivity was found in the fractions of degradation products.
Differences between the total and extractable amounts of intracellular radiolabeling showed that only 6 pmol of the labeling remained unextracted in wild-type tissues, compared to 149 pmol and 157 pmol in tCKX16 and tCKX7 tissues, respectively (data not shown). We presume that significant amounts of purine-like degradation products were fixed in macromolecules such as RNAs in the transformants, which are not extractable by the method employed. Thus, the dramatically higher amounts of nonextractable radioactivity in the CKX transformants further highlight their enhanced capacity to degrade cytokinins.
Unexpectedly, levels of radiolabeled iP nucleotides were found to be reduced in the extracts of the transformants (Table II), although cytokinin nucleotides are not, reportedly, substrates for CKX (Armstrong, 1994). This finding suggests that the possibility that cytokinin nucleotides could be substrates for CKX should be reassessed, in accordance with the finding of Galuszka et al. (2007) indicating that cytokinin nucleotides can indeed be substrates for certain AtCKX isoforms (AtCKX1, AtCKX2, and AtCKX3).
Furthermore, the lower amounts of iP nucleotides found in the transformants may reflect the fact that fewer cytokinins were available for nucleotide-forming reactions, especially via adenosine kinase (von Schwartzenberg et al., 1998, 2003). In addition, relatively large proportions of the iP nucleotides formed during the course of the incubation may have been metabolized by dephosphorylating enzymes (the activities of which were not analyzed in this study) in the transformants. Support for this assumption is provided by the finding that the relative amount of [2-3H]iPRMP dropped from 10% after 4 h to 2% after 20 h in tCKX16 cultures (Table II), indicating that most of the cytokinin nucleotide pool formed during early parts of the incubation were converted to cytokinin ribosides and bases, which were then available for CKX degradation at higher than wild-type rates.
After radiolabeling with [2-3H]iP or [2-3H]iPR, no Z-type cytokinins could be detected, indicating that trans-hydroxylation of free iP-type cytokinins by cytochrome P450 monooxygenase-like enzymes (Takei et al., 2004) is, if present at all, a rather slow process in Physcomitrella.
Endogenous Cytokinins and Influence of CKX Overexpression
In this work we present a comprehensive determination of intracellular and extracellular cytokinins in axenic cell cultures of a bryophyte and relate the results obtained to CKX overexpression. The UPLC-MS/MS measurements of endogenous cytokinins reflect their steady-state levels, as governed by their integrated rates of biosynthesis, interconversion, and breakdown.
The analyses revealed that Physcomitrella contains at least 20 different cytokinins, far more than previously reported in any mosses (Bopp, 1990). We detected isoprenoid cytokinins of iP, tZ, cZ, and DHZ type. While cZ-type cytokinins were predominant in the protonema tissue, iP-type cytokinins prevailed in the culture medium (Table III).
All groups of cytokinins occurred as bases, ribosides, and nucleotides (Supplemental Table S3, although levels of DHZRMP were mostly below the detection limit [data not shown]). The members of the Z family (both trans- and cis-isomers) were also present in the forms of O-glucosides (Fig. 3, A and B), indicating that the O-glycosylation mechanism, whereby the hormonal activity of Zs is “tuned” (Veach et al., 2003), is present in bryophytes as well as higher plants. Indeed, in Physcomitrella protonema tissues, tZ- and cZ-O-glucosides were the most abundant cytokinins, followed by nucleotides. In the extracellular fraction, Z-O-glucosides were less strongly represented.
Comparison of Endogenous Cytokinin Profiles from Algae, Moss, and Seed Plants
Ördög et al. (2004) carried out HPLC-MS-based studies on cytokinins in three genera of unicellular algae (Chlorophyta). Interestingly, the cytokinin profiles found for Protococcus, Chlorella, and Scenedesmus were similar to the one presented for Physcomitrella. However, concentrations of tZ-O-glucosides dominated over the cis-isomers. Aromatic forms (BA, mT, oT, pT) presented a considerable amount of cytokinins in unicellular algae (up to 65% in Protococcus). Like in Physcomitrella DHZ forms occurred only in traces and also no N-glucosides were found. Ivanova et al. (1992) reported for Chlamydomonas reinhardtii on the presence of high amounts of iP-type cytokinins (90%). tZ and DHZ forms were also present (cZ forms were not analyzed).
For multicellular green algae like Cladophora capensis and Ulva spec, a prevalence of both iP- and cZ-type cytokinins was found by HPLC-MS-based studies (Stirk et al., 2003). Again N-glucosides were absent. Thus, the moss Physcomitrella shares these features with other evolutionary primitive organisms. The dominance of cZ-type cytokinins in Physcomitrella and multicellular green algae seems to be one major difference to dicotyledonous seed plants such as Arabidopsis and tobacco, in which tZ-type cytokinins are usually dominant (Werner et al., 2001, 2003a, 2003b; Miyawaki et al., 2006).
Since tZ-type cytokinins have been shown to be generated by adenylate IPTs and cZ-type cytokinins by tRNA-IPTs in Arabidopsis (Miyawaki et al., 2006), the dominance of cZ cytokinins in Physcomitrella is consistent with the finding that no adenylate IPT genes could be identified so far in this plant (Yevdakova and von Schwartzenberg, 2007). Thus, cytokinin profiling data support the hypothesis that free cytokinins in Physcomitrella in contrast to seed plants might mainly derive from the release of cytokinin nucleotides during tRNA turnover.
In this work we also report for the first time in a bryophyte the occurrence of the aromatic cytokinins BA(R), mT, and oT (Fig. 3A), the biosynthesis of which is so far unclear.
Cytokinin Nucleotides in Culture Medium
To include polar cytokinin compounds in the analysis, we developed an extraction protocol involving freeze drying of the culture medium instead of the commonly used solid-phase extraction method. Remarkably, the predominant extracellular cytokinins were found to be nucleotides, predominantly represented by iPRMP (Fig. 3B; Supplemental Table S3). tZRMP and cZRMP were also found in the medium, but only in minor quantities.
The presence of extracellular cytokinin nucleotides was confirmed by Q-Tof MS, which provided both structural identification and highly accurate mass determinations (Supplemental Fig. S1). The detection of considerable concentrations of extracellular cytokinin nucleotides in Physcomitrella is in accordance with the previously reported occurrence of tZRMP and iPRMP in media of tobacco cell suspension cultures (Motyka et al., 2003).
The accumulation of extracellular nucleotides seems to be a rather slow process since during the short-term labeling experiments using [2-3H]iPR and [2-3H]iP no radioactive monophosphates were detected in the culture medium.
Since the tissue used for inoculation was washed and suspended in fresh medium at day 0, little or no nucleotides were detected at the starting point of the culture. During the following 20 d, the wild-type cultures accumulated 16 pmol iPRMP in 100 mL of medium, which we presume was released from the large pool of intracellular nucleotides (see Fig. 3, A and B). The alternative possibility, that they were formed from appropriate base or riboside precursors in the culture medium by external activities of adenine phosphoribosyl transferase or adenosine kinase, seems unlikely since these enzymes have not been detected in culture media to date. The fact that, like most other cytokinins, the internal iPRMP concentration decreased during the culture period (Fig. 3, A and B) suggests that cytokinin nucleotides are released from the cells. However, their transport mechanism is unclear.
Not All Detected Cytokinins Are Active in Physcomitrella Budding Bioassays
No detailed mass spectrometric analysis of natural cytokinins and concomitant assessment of their hormonal activity have previously been reported for Physcomitrella or any other bryophyte. One of the main advantages of mosses as experimental models for plant hormone research is that they can be grown in suspension culture and no artificial systems like callus or organ cultures need to be employed. We tested the biological activities of bases and/or ribosides of all cytokinin groups detected in the UPLC-MS/MS analysis (Fig. 3) using the cytokinin bioassay, with slight modifications, described for Funaria hygrometrica by Hahn and Bopp (1968). The main advantage of this assay is its high sensitivity and cytokinin specificity. Surprisingly, some of the detected cytokinins proved to be inactive in the Physcomitrella bioassay. The bases iP, tZ, and BA displayed the strongest bud-inducing capacity, but the activity of their corresponding ribosides was significantly lower (Fig. 5). These results are in agreement with results published by Whitaker and Kende (1974), who also found certain cytokinin ribosides to be less active than their corresponding bases in F. hygrometrica. The bud-inducing activities of mT, oT, and DHZ were all very weak in our assays.
Although the nucleotide iPRMP was the dominant cytokinin in the culture medium, it had no detectable bud-inducing activity, and neither did tZRMP and BARMP. However, it should be mentioned that tZRMP has been shown to be bound by the Arabidopsis AHK3 receptor, indicating that cytokinin nucleotides may have signaling functions (Spichal et al., 2004).
In addition, cZ and cZR, which are major cytokinins in Physcomitrella tissue (Fig. 3), exhibited no detectable bud-inducing activity. Thus, these and the other inactive cytokinins appear to play no role in the morphogenetic process of bud development in Physcomitrella (Fig. 5).
Surprisingly large amounts of cZ-type cytokinins, especially O-glycosylated riboside or base forms (Fig. 3, A and B), were found in Physcomitrella. Although cZ has been demonstrated to bind to receptors (Spichal et al., 2004; Yonekura-Sakakibara et al., 2004), the hormonal role of cZ cytokinins in plants remains unclear.
Assuming that the biogenesis of cZ-type cytokinins in Physcomitrella is generally, as recently demonstrated in Arabidopsis (Miyawaki et al., 2006), coupled to the isopentenylation of certain tRNAs containing cZ (and other hypermodified bases) adjacent to the anticodon, the possibility that the main function of cZ cytokinins is related to tRNA and the stabilization of codon-anticodon binding (Taller, 1994) cannot be completely excluded (although it seems unlikely). In this (unlikely) case the high amounts of cZ cytokinins would be simply a result of tRNA turnover without involvement in hormonal regulation. However, in contrast to cZ, other cytokinins found in tRNA, like iP, displayed strong activity in the budding bioassay (Fig. 5).
Cytokinin Deficiency and Phenotype in Physcomitrella
The main differences in endogenously produced cytokinins between wild-type and transgenic cultures were in the extracellular concentrations of iPR and iP, which were significantly reduced in the transformants (Fig. 3B). The reductions in the extracellular iP level were most pronounced at days 10 and 20. The findings of reduced iP and iPR contents in the medium are consistent with the results obtained in the labeling experiments using tritiated iP and iPR, in which rapid depletion of the extracellular label was observed (Fig. 2).
Concentrations of other endogenously produced cytokinins showed fewer obvious reductions and often only nonsignificant tendencies to decline. Indeed, both intracellular and extracellular cZR contents were even higher in the transformant cultures than in the wild-type cultures, implying that cZ-type cytokinins are probably resistant to AtCKX2 attack in this system, although cZ-type cytokinins are generally believed to be substrates for CKX (Armstrong, 1994). Enzymological data indicate that Z-O-glucosides, cZ-, and DHZ-type cytokinins are nonsubstrates or only very poor substrates for AtCKX2 (Galuszka et al., 2007), which could explain why cZ cytokinin levels were not reduced, or only slightly reduced, in tCKX7 and tCKX16 cultures (Fig. 3, A and B; see also Table III).
Our enzymatic studies on Physcomitrella CKX from crude extracts of untransformed wild-type tissue revealed that cZ is degraded at rates up to 7-fold and 4.5-fold higher than tZ and iP, respectively. In contrast, in assays with tCKX7 and tCKX16 protein extracts, cZ appears to be a much poorer CKX substrate than tZ and iP, and the order of CKX-catalyzed cleavage rates appears to be inverted: iP > tZ > cZ (S. Gajdošová, V. Motyka, and K. von Schwartzenberg, unpublished data).
The contents of the aromatic cytokinins BA, BAR, mT, and oT were also not reduced in tCKX7 and tCKX16 cultures (Fig. 3). This finding is in agreement with the low degradation capacity of CKX toward BA (Laloue and Fox, 1989; Armstrong, 1994; see also Fig. 2).
In the moss bud-induction assays, the number of buds induced on protonema is proportional to the hormonal activity in the culture medium (Bopp and Brandes, 1964; Hahn and Bopp, 1968). Comparing the budding responses to iP (tCKX7 < tCKX16 < wild type) with the [2-3H]iP degradation capacity (wild type < tCKX16 < tCKX7; Fig. 2B), it can be concluded that the reduced budding in the transformants is a consequence of increased cytokinin degradation (Supplemental Fig. S3).
In addition, extracellular iP and iPR are likely to be the main bud-inducing cytokinins in natural conditions in Physcomitrella, since concentrations of these hormones were most strongly reduced in cultures of the cytokinin-deficient transformants and both iP and iPR were found to have strong bud-inducing capacity in the Physcomitrella bioassay (Fig. 5). Extracellular iP and iPR also seem to have great importance for protonema development since cell morphology was altered under iP and iPR deficiency (Fig. 4; Supplemental Fig. S2). In cytokinin-overproducing Physcomitrella plants, the so-called ove mutants, major changes in hormone composition in the extracellular space have also been observed, principally accumulations of iP and iPR, which correlated with strong overproduction of buds (Wang et al., 1980; Schulz et al., 2001; von Schwartzenberg, 2006). Similar results have been obtained in an analysis of IPT-mediated cytokinin overproduction in Physcomitrella (Schulz et al., 2000). The finding that major changes occur in the culture medium of cytokinin-deficient as well as cytokinin-overproducing plants suggests that extracellular cytokinins are mainly responsible for developmental regulation in the evolutionarily primitive land plant Physcomitrella. The complete genome of Physcomitrella now being sequenced (Quatrano et al., 2007) provides the possibility of comparing genes of cytokinin signaling and metabolism with those of seed plants, hereby helping to complete our understanding of the evolution of cytokinin-mediated growth regulation.
MATERIALS AND METHODS
Plant Culture
Wild-type Physcomitrella patens (Hedw.) B.S.G. was maintained on solid agar medium using the ABC medium described by Knight et al. (1988)—containing 5 mm Ca(NO3)2, 0.035 mm FeSO4, 1.01 mm MgSO4, and 1.84 mm KH2PO4—supplemented with Hoagland trace element solution (1 mL/liter) and the vitamins p-aminobenzoic acid (1.8 μm), nicotine acid (8 μm), and thiamine HCl (1.5 μm). Agar (Select Agar; Gibco) was added to 1% (w/v) and the pH was adjusted to 6.5 by adding KOH.
For the determination of budding frequency, the less turbid Knop medium was used (Hahn and Bopp, 1968), consisting of 1.84 mm KH2PO4, 1.01 mm MgSO4, 3.35 mm KCl, and 2.72 mm Ca(NO3)2, in 1% agar (w/v), pH 6.4 (KOH).
Liquid cultures used for cytokinin profiling and metabolism studies were grown in a medium described by Wang et al. (1980), containing 0.359 mm Ca(NO3)2, 0.035 mm FeSO4, 1.01 mm MgSO4, 1.84 mm KH2PO4, 10 mm KNO3, and 5 mm diammonium tartrate. Trace elements and vitamins were added as indicated above for ABC medium. Liquid culture medium (400 mL) was inoculated with about 300 mg fresh weight (FW) of protonema filaments that had been freshly cut up using an Ultra-Turrax blender (IKA) into pieces each containing approximately 10 to 20 cells. Culture flasks (1,000 mL; Schott) with cotton wool bungs were aerated with water-saturated sterile air (approximately 600 mL/min). Cultures were grown at 15°C (maintenance) or 25°C under white light (Philips TLM) with a photon density of 100 μmol m−2 s−1 (400–700 nm) and 16-h/8-h (light/dark) photoperiods.
Construction of CKX-Overexpression Vector
cDNA of the AtCKX2 gene of Arabidopsis (Arabidopsis thaliana; accession no. AF303978) cloned into the vector pCR-Blunt II-topo (Invitrogen) was provided as a gift by T. Schmülling (FU-Berlin). The vector pBAS_GFP (Zeidler et al., 1999) containing the rice (Oryza sativa) actin1 promoter (accession no. S44221) controlling the GFP gene was provided as a gift by M. Zeidler (University of Giessen, Germany). pBAS_GFP was digested with NcoI and BsrGI, and the resulting 4,704-bp pBAS vector fragment lacking the GFP gene was blunted and dephosphorylated.
The AtCKX2 cDNA was isolated from the vector pCR-Blunt II-topo-AtCKX2 by digestion with KpnI, and the 1,600-bp AtCKX2 fragment was blunted and ligated into the pBAS vector backbone. Since the resulting vector pBAS_AtCKX2 did not contain a plant-selective marker, the entire expression cassette (3,330 bp) with the actin1 promoter, AtCKX2, and terminator was isolated from pBAS_AtCKX2 by XbaI/HindIII digestion and then blunt-end ligated into the NdeI site of the vector pHP23_ΔBamHI_ΔSalI containing a 35S-nptII selection cassette (Paszkowski et al., 1988). The resulting vector, pHP_act1_AtCKX2 (7,680 bp), was checked by PCR, restriction analysis, and partial sequencing.
Generation of Transgenic Plants
The construct pHP_act1_AtCKX2 was transferred into Physcomitrella protoplasts derived from liquid cultures by PEG-mediated transformation according to Schaefer et al. (1991) using circular DNA. Of a total of 30 transformants, the stable strains tCKX7 and tCKX16 were chosen after several selection cycles on selective and nonselective media for further characterization.
PCR Analysis of Transformants
The presence of the construct pHP_act1_AtCKX2 in genomic DNA of the Physcomitrella transformants tCKX7 and tCKX16 was demonstrated by PCR using the primers 5′-ATCATCAGCAAGGTTATTGACAC-3′ and 5′-TCATCGCCGACATACGATTG-3′; at an annealing temperature of 55°C, a 307-bp fragment of AtCKX2 was amplified.
In Vivo Metabolism Studies
The in vivo metabolization of the radiolabeled cytokinins [2-3H]BA,[2-3H]iP, and [2-3H]iPR was analyzed as described by von Schwartzenberg et al. (2003). The tritiated substrates used for this purpose were obtained from the Isotope Laboratory, Institute of Experimental Botany AS CR, Prague, Czech Republic.
CKX Enzyme Assay
The CKX from Physcomitrella cells (plant material equivalent to approximately 1.3–1.5 g DW) was extracted and partially purified using the method of Chatfield and Armstrong (1986) as modified by Motyka et al. (2003). The CKX from the media of Physcomitrella cultures (equivalent to 265 mL) was precipitated by the addition of solid ammonium sulfate directly to the medium to 80% saturation. The concentrations of proteins in the enzyme preparations from both cells and media were determined according to the method of Bradford (1976) using bovine serum albumin as a standard.
The CKX activity was determined by in vitro assays in which the conversion of [2-3H]iP to [2-3H]adenine in the copper-imidazole-sensitized technique described by Chatfield and Armstrong (1987) and Motyka et al. (1996) was measured. The assay mixture (50 μL final volume) included 100 mm imidazole buffer (pH 6.0) containing 25 mm sodium acetate and 5 mm CuCl2, 2 μm substrate ([2-3H]iP, 7.4 TBq mol−1), and enzyme preparations (equivalent to 0.45–0.95 mg protein g−1 FW for cells or 0.45–1.2 mg protein mL−1 for culture media). After incubation at 37°C, the reaction was terminated and the substrate was separated from the product of the enzyme reaction by HPLC as described elsewhere (Gaudinová et al., 2005).
Each CKX determination was performed in two independent biological samples and repeated three times. Results of one representative determination are presented. Statistical variation of results are expressed as the average ± sd.
Cytokinin Analysis by UPLC-MS/MS
Samples of both the culture media (100 mL) and tissues were freeze-dried and stored at −20°C until LC-MS analysis. The procedure used for cytokinin analysis was a modified form of the method described by Faiss et al. (1997). Freeze-dried plant material was homogenized in liquid nitrogen and extracted in ice-cold 70% (v/v) ethanol. Deuterium-labeled cytokinin internal standards (Olchemim Ltd.) were added, each at 5 pmol per sample, to evaluate the recovery during purification and to validate the determination. The standards were [2H5]tZ, [2H5]tZR, [2H5]tZ9G, [2H5]tZOG, [2H5]tZROG, [2H5]tZRMP, [2H3]DHZ, [2H3]DHZR, [2H3]DHZ9G, [2H3]DHZOG, [2H3]DHZROG, [2H3]DHZRMP, [2H6]iP, [2H6]iPR, [2H6]iP9G, [2H6]iPRMP, [2H7]BA, [2H7]BAR, [2H7]BA9G, [2H7]BARMP, [15N4]mT, and [15N4]oT (see Supplemental List S1 for definitions of abbreviations). All topolins were quantified using internal deuterium standards for [15N4]mT and [15N4]oT since no other labeled standards were available. Therefore, the values for other topolin metabolites may be subject to errors originating from imperfect internal standardization. After 3 h of extraction, the homogenate was centrifuged (15,000g, 15 min at 4°C) and the pellets were reextracted. The combined supernatants were concentrated to approximately 1.0 mL by rotary evaporation under vacuum at 35°C, then diluted to 20 mL with ammonium acetate buffer (40 mm, pH 6.5). The extracts were purified using a combined DEAE-Sephadex (Sigma-Aldrich; 1.0 × 5.0 cm)-octadecylsilica (0.5 × 1.5 cm) column and immunoaffinity chromatography based on wide-range specific monoclonal antibodies against cytokinins (Faiss et al., 1997). This resulted in three fractions: (1) the free bases and 9-glycosides (fraction B), (2) a nucleotide fraction, and (3) an O-glucoside fraction. These fractions were each evaporated to dryness and dissolved in 20 μL of the mobile phase used for quantitative analysis.
The cytokinin fractions were analyzed using an ACQUITY UPLC ultra-performance liquid chromatograph (Waters), equipped with a BEH C18 (1.7 μm; 2.1 × 150 mm) column, linked to a Quattro micro API (Waters MS Technologies) triple quadrupole mass spectrometer equipped with an electrospray interface. The purified samples were dissolved in 15 μL MeOH/H2O (30/70, v/v) and 10 μL of each sample was injected into the chromatographic system. The analytes were eluted with a 10-min binary linear gradient, composed of 15 mm ammonium formate (pH 4.0, A) and methanol (B) starting at an A:B ratio of 1:9 (v/v) and finishing at a 1:1 ratio of A:B (flow rate 0.25 mL/min, column temperature 40°C), with retention times for the monitored compounds ranging from 2.50 to 6.50 min.
The analytes were quantified by multiple reaction monitoring of [M+H]+ and the appropriate product ion. For selective MRM experiments, optimal conditions were as follows: capillary voltage 0.6 kV, source/desolvation gas temperature 100°C/350°C, cone/desolvation gas 2.0/550 L/h, LM/HM resolution 12.5, ion energy 1 0.3 V, ion energy 2 1.5 V, entrance 2.0 V, exit 2.0 V, multiplier 650 eV. The dwell time, cone voltage, and collision energy corresponding to exact diagnostic transition were optimized for each cytokinin. On the basis of the observed retention times, which appeared to be sufficiently constant, the chromatographic run was split into eight retention windows. The dwell time of each MRM channel was calculated to provide 16 scan points per peak, during which time the inter channel delay was 0.1 s. In MRM mode, the limit of detection for most of the cytokinins was lower than 5.0 fmol and the linear range was at least five orders of magnitude. The identity of all measured cytokinin metabolites was verified by comparison of the mass spectra and chromatographic retention times with those of authentic standards.
Identification of iPRMP by Exact Mass Determination
A CapLC module (Waters) capillary liquid chromatography system equipped with a reversed-phase (Symmetry C18, 0.3×150 mm, 5 μm; Waters) column coupled to a hybrid Q-Tof micro mass analyzer (Waters MS Technologies) was used for high resolution identification and confirmation of iPRMP. Following injection, cytokinins were eluted with a 25-min binary linear gradient, again composed of 15 mm ammonium formate (pH 4.0, A) and methanol (B) starting at an A:B ratio of 1:9 (v/v) and finishing at a 1:1 ratio of A:B, but with a flow-rate of 5 μL/min and column temperature of 35°C. Electrospray ionization in the positive ion mode was performed using the following parameters: source block/desolvation temperature, 90°C/200°C; capillary/cone voltage, 2,500/30 V; and spray/cone gas flow (N2), 50/250L/h. In the full-scan mode, data were acquired in the mass range of 50 to 500 D, with a cycle time of 28 μs, a scan time of 1.0 s, and a collision energy of 4 V. For the MS/MS experiments, analytes were fragmented with the collision cell filled with argon gas and collision energies of 15, 20, and 25 V. For the exact mass determination experiments, a lock spray was used for external calibration with a mixture of 0.1 m NaOH/10% formic acid (v/v) and acetonitrile (1:1:8 by volume) as a reference. Accurate masses were calculated and used for the determination of the elementary composition and structure of the analytes with fidelity ≥5 ppm. All data were processed by the QuanLynx program included in the MassLynx software package (version 4.0; Waters).
Physcomitrella Budding Bioassay
Undifferentiated protonemic tissues of 7-d-old Physcomitrella liquid cultures were rinsed with sterile water and used to inoculate petri dishes with Knop-agar medium (Hahn and Bopp, 1968) containing cytokinins. After 6 d of growth under light (see above) at 25°C bud formation was recorded by microscopic observation using an inverse microscope.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Mass spectrometric identification of IPRMP in culture medium.
Supplemental Figure S2. Distribution of protonema cell dimensions in the wild type, tCKX7, and tCKX16.
Supplemental Figure S3. Budding response of the wild type, tCKX7, and tCKX16 to exogenous iP.
Supplemental Table S1. Intracellular cytokinin concentrations, dataset for Figure 3A.
Supplemental Table S2. Extracellular cytokinin concentrations, dataset for Figure 3B.
Supplemental Table S3. Cumulative concentrations for various cytokinin groups, intracellular and extracellular.
Supplemental List S1. Abbreviations of cytokinin metabolites.
Supplementary Material
Acknowledgments
We thank Petra Amakorová (Olomouc), Marie Korecká (Prague), Susanne Bringe, Jutta Krüger, and Vera Schwekendiek (Hamburg) for skillful technical assistance. M.F. acknowledges funding from the University of Hamburg's “Graduiertenförderung” program. The authors thank Mark Held (University of Waterloo, Canada) and Heinz Hahn (University of Hamburg) for critically reading a previous version of this manuscript, and Peter Schulz and Radka Vanková (Prague) for contributions in earlier parts of this project. The authors further acknowledge Thomas Schmülling (FU-Berlin) for providing the AtCKX2 cDNA.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. DFG Schw687/4); the Ministry of Education, Youth and Sports of the Czech Republic (grant no. MSM6198959216); the Grant Agency of the Czech Republic (grant no. 522/06/0703); and the Grant Agency of the Academy of Sciences of the Czech Republic (grant no. IAA600380701).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Klaus von Schwartzenberg (kvschwartzenberg@botanik.uni-hamburg.de).
The online version of this article contains Web-only data.
References
- Armstrong DJ (1994) Cytokinin oxidase and the regulation of cytokinin degradation. In DWS Mok, MC Mok, eds, Cytokinins: Chemistry, Activity, and Function. CRC Press, Boca Raton, FL, pp 139–154
- Bopp M (1990) Hormones of the moss protonema. In RN Chopra, SC Bhatla, eds, Bryophyte Development: Physiology and Biochemistry. CRC Press, Boca Raton, FL, pp 55–77
- Bopp M, Brandes H (1964) Versuche zur Analyse der Protonemaentwicklung der Laubmoose. II. Über den Zusammenhang zwischen Protonemadifferenzierung und Kinetinwirkung bei der Bildung von Moosknospen. Planta 62 116–136 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Chatfield JM, Armstrong DJ (1986) Regulation of cytokinin oxidase activity in callus tissues of Phaseolus vulgaris L. cv Great Northern. Plant Physiol 80 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield JM, Armstrong DJ (1987) Cytokinin oxidase from Phaseolus vulgaris callus tissues: enhanced in vitro activity of the enzyme in the presence of copper-imidazole complexes. Plant Physiol 84 726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM (1997) Cytokinin biosynthesis and interconversion. Physiol Plant 101 665–673 [Google Scholar]
- Cove D, Bezanilla M, Harries P, Quatrano R (2006) Mosses as model systems for the study of metabolism and development. Annu Rev Plant Biol 57 497–520 [DOI] [PubMed] [Google Scholar]
- Faiss M, Zalubilová J, Strnad M, Schmülling T (1997) Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J 12 401–415 [DOI] [PubMed] [Google Scholar]
- Frebort I, Sebela M, Galuszka P, Werner T, Schmülling T, Pec P (2002) Cytokinin oxidase/cytokinin dehydrogenase assay: optimized procedures and applications. Anal Biochem 306 1–7 [DOI] [PubMed] [Google Scholar]
- Galuszka P, Frébort I, Sebela M, Sauer P, Jacobsen S, Pec P (2001) Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals. Eur J Biochem 268 450–461 [DOI] [PubMed] [Google Scholar]
- Galuszka P, Popelková H, Werner T, Frébortová J, Popíšilová H, Mik V, Köllmer I, Schmülling T, Frébort I (2007) Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J Plant Growth Regul 26 255–267 [Google Scholar]
- Gaudinová A, Dobrev PI, Šolcová B, Novák O, Strnad M, Friedecký D, Motyka V (2005) The involvement of cytokinin oxidase/dehydrogenase and zeatin reductase in regulation of cytokinin levels in pea (Pisum sativum L.) leaves. J Plant Growth Regul 24 188–200 [Google Scholar]
- Hahn H, Bopp M (1968) A cytokinin test with high specificity. Planta 83 115–118 [DOI] [PubMed] [Google Scholar]
- Houba-Hérin N, Pethe C, d'Alayer J, Laloue M (1999) Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. Plant J 17 615–626 [DOI] [PubMed] [Google Scholar]
- Ivanova M, Todoruv I, Pashankov P, Kostova L, Kaminek M (1992) Estimation of cytokinins in the unicellular green algae Chlamydomonas reinhardtii Dang. In M Kaminek, DWS Mok, E Zazimalova, eds, Physiology and Biochemistry of Cytokinins in Plants. SPB Academic Publishing, The Hague, The Netherlands, pp 483–485
- Kakimoto T (2001) Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol 42 677–685 [DOI] [PubMed] [Google Scholar]
- Kieber JJ (2002) Cytokinins. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi: 10.1199/tab.0063, www.aspb.org/publications/arabidopsis/
- Knight CD, Cove DJ, Boyd PJ, Ashton NW (1988) The isolation of biochemical and developmental mutants in Physcomitrella patens. In JM Glime, ed, Methods in Bryology: Proceedings of the Bryology Methods Workshop, Mainz. Hattori Botanical Laboratory, Nichinan, Japan, pp 47–58
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445 652–655 [DOI] [PubMed] [Google Scholar]
- Laloue M, Fox JE (1989) Cytokinin oxidase from wheat: partial purification and general properties. Plant Physiol 90 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letham DS, Palni LMS, Tao GQ, Gollnow BI, Bates CM (1983) Regulators of cell division in plant tissues XXIX. The activities of cytokinin glucosides and alanine conjugates in cytokinin bioassay. J Plant Growth Regul 2 103–115 [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52 89–118 [DOI] [PubMed] [Google Scholar]
- Morris RO, Bilyeu KD, Laskey JG, Cheikh NN (1999) Isolation of a gene encoding a glycosylated cytokinin oxidase from maize. Biochem Biophys Res Commun 255 328–333 [DOI] [PubMed] [Google Scholar]
- Motyka V, Faiss M, Strnad M, Kamínek M, Schmülling T (1996) Changes in cytokinin content and cytokinin oxidase activity in response to derepression of ipt gene transcription in transgenic tobacco calli and plants. Plant Physiol 112 1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyka V, Vaňková R, Čapková V, Petrášek J, Kamínek M, Schmülling T (2003) Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiol Plant 117 11–21 [Google Scholar]
- Ördög V, Stirk WA, van Staden J, Novák O, Strnad M (2004) Endogenous cytokinins in three genera of microalgae from the Chlorophyta. J Phycol 40 88–95 [Google Scholar]
- Paces V, Werstiuk E, Hall RH (1971) Conversion of N6-(Δ2-isopentenyl)adenosine to adenosine by enzyme activity in tobacco tissue. Plant Physiol 48 775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski J, Baur M, Bogucki A, Potrykus I (1988) Gene targeting in plants. EMBO J 7 4021–4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatrano RS, McDaniel SF, Khandelwal A, Perroud PF, Cove DJ (2007) Physcomitrella patens: mosses enter the genomic age. Curr Opin Plant Biol 10 182–189 [DOI] [PubMed] [Google Scholar]
- Reski R, Abel WO (1985) Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165 354–358 [DOI] [PubMed] [Google Scholar]
- Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57 431–449 [DOI] [PubMed] [Google Scholar]
- Schaefer D, Zryd JP, Knight CD, Cove DJ (1991) Stable transformation of the moss Physcomitrella patens. Mol Gen Genet 226 418–424 [DOI] [PubMed] [Google Scholar]
- Schmülling T, Werner T, Riefler M, Krupková E, Manns IB (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116 241–252 [DOI] [PubMed] [Google Scholar]
- Schulz P, Reski R, Maldiney R, Laloue M, von Schwartzenberg K (2000) Kinetics of cytokinin production and bud formation in Physcomitrella: analysis of wild type, a developmental mutant and two of its ipt transgenics. J Plant Physiol 156 768–774 [Google Scholar]
- Schulz PA, Hofmann AH, Russo VE, Hartmann E, Laloue M, von Schwartzenberg K (2001) Cytokinin overproducing ove mutants of Physcomitrella patens show increased riboside to base conversion. Plant Physiol 126 1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spichal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3 differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45 1299–1305 [DOI] [PubMed] [Google Scholar]
- Stirk WA, Novák O, Strnad M, van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41 13–24 [Google Scholar]
- Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279 41866–41872 [DOI] [PubMed] [Google Scholar]
- Taller BJ (1994) Distribution, biosynthesis and function of cytokinins in tRNA. In DWS Mok, MC Mok, eds, Cytokinins: Chemistry, Activity and Function. CRC Press, Boca Raton, FL, pp 101–112
- Veach YK, Martin RC, Mok DWS, Malbeck J, Vankova R, Mok MC (2003) O-Glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131 1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwartzenberg K (2006) Moss biology and phytohormones—cytokinins in Physcomitrella. Plant Biol 8 382–388 [DOI] [PubMed] [Google Scholar]
- von Schwartzenberg K, Kruse S, Reski R, Moffatt B, Laloue M (1998) Cloning and characterization of an adenosine kinase from Physcomitrella involved in cytokinin metabolism. Plant J 13 249–257 [DOI] [PubMed] [Google Scholar]
- von Schwartzenberg K, Pethe C, Laloue M (2003) Cytokinin metabolism in Physcomitrella patens—differences and similarities to higher plants. Plant Growth Regul 39 99–106 [Google Scholar]
- Wang TL, Cove DJ, Beutelmann P, Hartmann E (1980) Isopentenyladenine from mutants of the moss, Physcomitrella patens. Phytochemistry 19 1103–1105 [Google Scholar]
- Wang Y, Zhang W, Cao J, McElroy D, Wu R (1992) Characterization of cis-acting elements regulating transcription from the promoter of a constitutively active rice actin gene. Mol Cell Biol 12 3399–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Hanus J, Holub J, Schmülling T, Van Onckelen H, Strnad M (2003. a) New cytokinin metabolites in IPT transgenic Arabidopsis thaliana plants. Physiol Plant 118 127–137 [DOI] [PubMed] [Google Scholar]
- Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T (2006) New insights into the biology of cytokinin degradation. Plant Biol 8 371–381 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Onckelen HV, Schmülling T (2003. b) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 152532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker BD, Kende H (1974) Bud formation in Funaria hygrometrica: a comparison of the activities of three cytokinins with their ribosides. Planta 121 93–96 [DOI] [PubMed] [Google Scholar]
- Whitty CD, Hall RH (1974) A cytokinin oxidase in Zea mays. Can J Biochem 52 789–799 [DOI] [PubMed] [Google Scholar]
- Yevdakova NA, von Schwartzenberg K (2007) Characterisation of a prokaryote-type tRNA-isopentenyltransferase gene from the moss Physcomitrella patens. Planta 226 683–695 [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H (2004) Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol 134 1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler M, Hartmann E, Hughes J (1999) Transgene expression in the moss Ceratodon purpureus. J Plant Physiol 154 641–650 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.