Abstract
Reactive oxygen species (ROS) are responsible for mediating cellular defense responses in plants. Controversy has existed over the origin of ROS in plant defense. We have isolated a novel extracellular peroxidase gene, CaPO2, from pepper (Capsicum annuum). Local or systemic expression of CaPO2 is induced in pepper by avirulent Xanthomonas campestris pv vesicatoria (Xcv) infection. We examined the function of the CaPO2 gene in plant defense using the virus-induced gene silencing technique and gain-of-function transgenic plants. CaPO2-silenced pepper plants were highly susceptible to Xcv infection. Virus-induced gene silencing of the CaPO2 gene also compromised hydrogen peroxide (H2O2) accumulation and hypersensitive cell death in leaves, both locally and systemically, during avirulent Xcv infection. In contrast, overexpression of CaPO2 in Arabidopsis (Arabidopsis thaliana) conferred enhanced disease resistance accompanied by cell death, H2O2 accumulation, and PR gene induction. In CaPO2-overexpression Arabidopsis leaves infected by Pseudomonas syringae pv tomato, H2O2 generation was sensitive to potassium cyanide (a peroxidase inhibitor) but insensitive to diphenylene iodonium (an NADPH oxidase inhibitor), suggesting that H2O2 generation depends on peroxidase in Arabidopsis. Together, these results indicate that the CaPO2 peroxidase is involved in ROS generation, both locally and systemically, to activate cell death and PR gene induction during the defense response to pathogen invasion.
Upon recognition of pathogens, plants activate a battery of defense responses, including the oxidative burst, the hypersensitive response (HR), cell wall fortification, and defense-related protein synthesis (Hammond-Kosack and Jones, 1996; Lamb and Dixon, 1997; Jones and Dangl, 2006). One of the most rapid defense reactions to pathogen attack is the oxidative burst, which leads to the transient production of large amounts of reactive oxygen species (ROS), including superoxide (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (·OH; Mehdy, 1994; Lamb and Dixon, 1997; Kawano, 2003; Laloi et al., 2004). The oxidative burst occurs during the HR in plants following the perception of pathogen avirulence signals (Hammond-Kosack and Jones, 1996; Lamb and Dixon, 1997). The rapid generation of O2− or its dismutation product H2O2 at the onset of the HR has been documented in many plant-pathogen interactions (Doke, 1983; Grant et al., 2000). ROS produced in the oxidative burst not only protect against invading pathogens but also function as signaling molecules to activate plant defense responses (Tenhaken et al., 1995; Jabs, 1999). During the defense response in plant cells, ROS can inhibit pathogens by strengthening host cell walls via the oxidative cross-linking of glycoproteins, such as the Pro-rich protein (Bradley et al., 1992), or by directly killing pathogens (Levine et al., 1994).
Several enzymes have been implicated in the generation of ROS during defense responses in a number of plant-pathogen interactions. An NADPH-dependent oxidase system was initially found in mammalian neutrophils (Babior, 1984; Apel and Hirt, 2004). NADPH oxidase, known as the respiratory burst oxidase, catalyzes the production of O2− by the one-electron reduction of molecular oxygen using NADPH as an electron donor. The rapid transformation of O2− into H2O2 during plant-pathogen interactions was first observed in potato (Solanum tuberosum) tubers infected with an avirulent race of Phytophthora infestans (Doke, 1983; Park and Doke, 2005). Rboh (respiratory burst oxidase homologs) genes encoding gp91phox (the catalytic subunit of the NADPH oxidase of phagocytes) have been found in rice (Oryza sativa; Groom et al., 1996), Nicotiana benthamiana (Yoshioka et al., 2003), and Arabidopsis (Arabidopsis thaliana; Desikan et al., 1998; Keller et al., 1998; Torres et al., 1998; Laloi et al., 2004; Torres and Dangl, 2005). Interestingly, plant NADPH oxidases have an additional N-terminal calcium-binding EF hand, which is absent from the phagocyte NADPH oxidases, suggesting direct activation by Ca2+ ions in plant cells in contrast to mammalian cells (Keller et al., 1998). Knockout mutations of Atrboh genes eliminate ROS production in the defense response of Arabidopsis to avirulent pathogens (Torres et al., 2002). Pathogen-induced, NADPH oxidase-derived ROS were recently demonstrated to play a role in suppressing the spread of cell death in Arabidopsis (Torres et al., 2005).
In addition to plant NADPH oxidases, peroxidases have been proposed as alternative producers of ROS (Apel and Hirt, 2004; Bindschedler et al., 2006). Peroxidases catalyze the oxidoreduction of various substrates using H2O2. Many peroxidases localize to vascular and apoplastic compartments. Notably, peroxidases in the cell wall can generate apoplastic H2O2 at neutral to basic pH in the presence of reductants in plant cells (Bolwell et al., 1998). In French bean (Phaseolus vulgaris) suspension-cultured cells treated with a cell wall elicitor from Colletotrichum lindemuthianum, cell wall peroxidase rather than NADPH oxidase has been proposed to be the major source of ROS (Bolwell and Wojtaszek, 1997; Bolwell et al., 1998, 1999). In this system, elicitors are recognized by putative receptors located on the plasma membrane to activate ion channels in plant cells. The movement of H+ and other ions such as Ca2+, K+, and Cl− may contribute to a transient alkalinization of the extracellular matrix, ultimately activating the pH-dependent cell wall peroxidase to generate ROS (Zimmerlin et al., 1994).
ROS from the oxidative burst were proposed to orchestrate the plant defense response and hypersensitive cell death during incompatible plant-pathogen interactions (Levine et al., 1994). Since then, there has been increasing support for a key role of ROS as triggers of cell death. Recently, transgenic plants with perturbed levels of cellular antioxidants have demonstrated the important role of ROS in different plant cell death events (Lorrain et al., 2003). Arabidopsis mutants of the atrbohD and atrbohF genes exhibit reduced hypersensitive cell death and an absence of ROS in response to avirulent Pseudomonas syringae (Torres et al., 2002). Similarly, Nbrboh-silenced N. benthamiana plants not only cannot accumulate H2O2 but also are compromised for HR activation following P. infestans infection (Yoshioka et al., 2003). Moreover, ROS have also been proposed to mediate a reiterative signal network underlying systemic acquired resistance (SAR; Alvarez et al., 1998; Lee and Hwang, 2005). The infection of lower pepper (Capsicum annuum) leaves with an avirulent strain of Xanthomonas campestris pv vesicatoria (Xcv) induces SAR in the uninoculated upper leaves (Lee and Hwang, 2005). SAR is associated with a rapid increase in the oxidative burst, with the generation of O2− and the subsequent accumulation of H2O2 in plant tissues (Lamb and Dixon, 1997). For instance, the SAR response of pepper plants is accompanied by a systemic microoxidative burst that generates H2O2 and a systemic expression of defense-related genes in uninoculated leaves (Lee and Hwang, 2005). ROS induce the coordinate expression of a set of so-called SAR genes. There are indications that H2O2 acts as an intercellular or intracellular secondary messenger for the systemic induction of various defense genes in plants (Orozco-Cárdenas et al., 2001). More recently, NADPH oxidase has been demonstrated to mediate the rapid, systemic generation of ROS in response to virus infection in Arabidopsis (Love et al., 2005).
In this study, we have isolated and functionally characterized a novel extracellular peroxidase gene pepper PEROXIDASE2 (CaPO2) that is implicated in disease resistance. We used the virus-induced gene silencing (VIGS) technique in pepper (Baulcombe, 1999) and ectopic expression in Arabidopsis (Clough and Bent, 1998) as efficient forward genetic approaches to define the functions of the CaPO2 gene in plant defense. CaPO2-silenced pepper plants were highly susceptible to infection by Xcv. Moreover, silencing of the CaPO2 gene not only compromised the oxidative burst and the HR in primary infected leaves (leading to a local response) but also microoxidative bursts and micro-HR in secondary leaves elsewhere in the same plant (leading to a systemic response) during infection by avirulent Xcv. In contrast, transgenic Arabidopsis plants that constitutively overexpressed the CaPO2 gene exhibited enhanced bacterial disease resistance, as well as the oxidative burst and the HR.
RESULTS
The CaPO2 cDNA Encodes Extracellular Peroxidase
The CaPO2 cDNA was isolated from a cDNA library made from pepper leaves infected with the avirulent strain Bv5-4a of Xcv by using a macro-cDNA array method (Jung and Hwang, 2000). The CaPO2 cDNA consists of 1,120 bp, including a 19 bp poly-A tail, and codes for a protein of 322 amino acids with a predicted molecular mass of 34.6 kD and a pI of 8.04. The translated CaPO2 amino acid sequence was used as a query for a database search (http://www.ncbi.nlm.nih.gov/blast/), which revealed that it showed homology to extracellular peroxidases. The putative amino acid sequence encoded by the CaPO2 cDNA clone was 85% identical to lignin-forming anionic peroxidase of wood tobacco (Nicotiana sylvestris; accession no. B56555) and 33% identical to CaPO1 (accession no. AAL35364; Do et al., 2003). Computational analysis of the CaPO2 peptide sequence using PROSITE (www.expasy.org/prosite) revealed that it possesses a proximal heme-binding ligand (H-196), a peroxidase active site signature, and four conserved disulphide bridges (Supplemental Fig. S1).
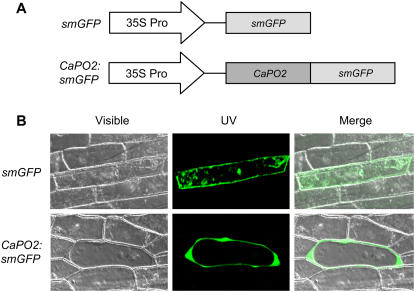
Computational analysis of the predicted protein sequence revealed that CaPO2 is likely to be a secreted protein (PSORT, 82%; TargetP, 94.8%) and that it has a signal peptide cleavage site between 27-Ala(A) and 28-Gln(Q) (Supplemental Fig. S1). To determine the subcellular localization of the CaPO2 protein, the soluble-modified GFP (smGFP) gene was fused to CaPO2 under the control of the cauliflower mosaic virus (CaMV) 35S promoter (Fig. 1A). The 35S:CaPO2:smGFP fusion construct and a 35S:smGFP control were introduced into onion (Allium cepa) epidermal cells by particle bombardment. As shown in Figure 1B, the CaPO2:smGFP fusion protein was localized in the extracellular matrix, while the control smGFP was uniformly distributed throughout the cell.
Figure 1.
Analysis of the subcellular localization of the CaPO2 protein by transient expression of the CaPO2:smGFP construct in onion epidermal cells. A, Schematic of the smGFP-tagged CaPO2 construct and of a control construct. The smGFP gene was fused to the 3′ region of the CaPO2 gene. B, Transient expression of smGFP or CaPO2:smGFP in onion epidermal cells was detected by confocal laser-scanning microscopy 24 h after biolistic transformation.
CaPO2 Is Strongly Induced in Leaves Infected by Avirulent Xcv
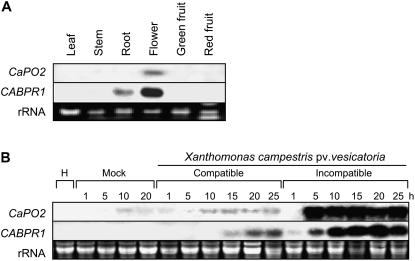
The expression of the pepper CaPO2 gene in different organs was examined by RNA gel-blot analysis. As shown in Figure 2A, CaPO2 transcripts were only faintly detected in the floral tissue of healthy pepper plants. However, they were not detected in leaves, stems, or roots, or in green or red fruits. This observation suggests that the CaPO2 gene is not constitutively expressed in healthy plant organs, except for the flower. RNA gel-blot analysis of the CaPO2 gene was performed to determine whether the gene is induced in pepper leaves during compatible and incompatible interactions with Xcv (Fig. 2B). The CaPO2 and CaBPR1 (pepper basic PR1 protein) genes were strongly induced in leaves inoculated with the avirulent (incompatible) strain Bv5-4a. In particular, the induction of CaPO2 could be detected as early as 5 h after Bv5-4a infection, i.e. before the appearance of the HR. However, CaPO2 transcripts were only faintly detected in mock-inoculated leaves (10 mm MgCl2) or in leaves inoculated with the virulent (compatible) strain Ds1. The same RNA samples were probed with the CaBPR1 as a positive control. This result suggests that the CaPO2 gene is strongly induced during the resistance response of pepper plants to Xcv infection.
Figure 2.
RNA gel-blot analyses of expression of the CaPO2 and CaBPR1 genes in pepper plants. The membranes were hybridized with 32P-dCTP labeled CaPO2 and CaBPR1 probes. Equal loading of total RNA (20 μg per lane) was verified by visualizing rRNA on a gel stained with ethidium bromide. A, Organ-specific expression of the CaPO2 and CaBPR1 genes in pepper plants. B, Expression of CaPO2 and CaBPR1 in pepper leaves at various time points after inoculation with the virulent strain Ds1 (compatible) and the avirulent strain Bv5-4a (incompatible) of Xcv at the six-leaf stage. H, Healthy leaves; Mock, leaves treated with 10 mm MgCl2.
Avirulent Xcv Infection Induces Oxidative Bursts and the HR in Pepper Plants
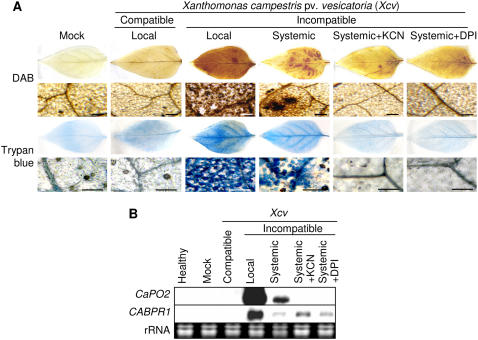
To dissect the roles of extracellular CaPO2 peroxidase in H2O2 generation and in the HR during the defense response of pepper plants to Xcv, we stained leaves with diaminobenzidine (DAB; a histochemical reagent for H2O2) and with trypan blue to detect the micro-HR. Strong and differential staining with DAB and trypan blue was observed in both primary and secondary leaves 24 h after inoculation of primary leaves with the Xcv avirulent strain Bv5-4a. Intriguingly, infiltration of secondary leaves with potassium cyanide (KCN; a peroxidase inhibitor) or diphenylene iodonium chloride (DPI; an NADPH oxidase complex inhibitor) effectively inhibited the subsequent induction of the microoxidative bursts and of micro-HR in uninoculated secondary leaves (Fig. 3A). Notably, CaPO2 transcripts in secondary leaves were also eliminated by KCN or DPI treatment, although the pepper pathogenesis-related gene (CaBPR1) was induced under these conditions (Fig. 3B). Thus, we hypothesize that the extracellular peroxidase CaPO2 and NADPH oxidase are both required for systemic microbursts and micro-HR in pepper leaves.
Figure 3.
Peroxidase- and NADPH oxidase-dependent oxidative burst and HRs in pepper plants. A, DAB and trypan blue staining of the primary and secondary pepper leaves at different times after inoculation of primary leaves with the avirulent strain Bv5-4a (108 cfu mL−1) of Xcv. Secondary leaves were also simultaneously infiltrated with either 1 mm KCN (peroxidase inhibitor) or 2.5 μm DPI (NADPH oxidase complex inhibitor) immediately after Xcv inoculation. Xcv-infected local leaves were harvested for DAB and trypan blue staining 1 and 24 h after inoculation, respectively; however, uninoculated systemic leaves were stained with both agents 24 h after inoculation. Bars = 200 μm. B, RNA gel-blot analysis of expression of CaPO2 and CaBPR1 in primary and secondary pepper leaves 24 h after inoculation with the Xcv avirulent strain Bv5-4a at the six-leaf stage. The membrane was successively hybridized with 32P-dCTP-labeled CaPO2 and CaBPR1 probes. Local, Xcv-inoculated leaves; Systemic, uninoculated leaves distant from the Xcv infection site.
Silencing of the CaPO2 Gene in Pepper Increases Disease Susceptibility Accompanied by Compromised Defense-Related Gene Expression
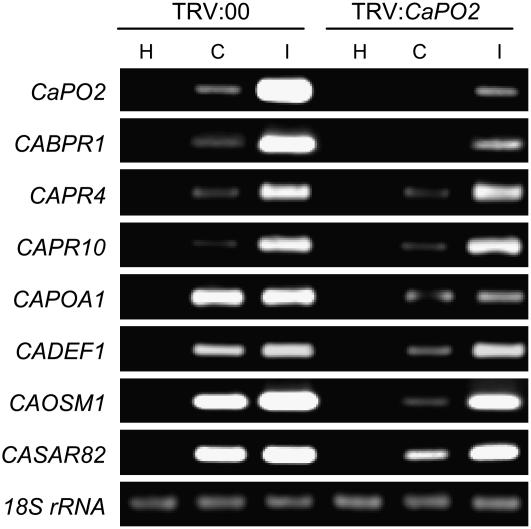
We observed that the CaPO2 gene was strongly induced during the incompatible interaction of pepper plants with the Xcv avirulent strain Bv5-4a (Fig. 2B). To examine the effect of loss of function of the CaPO2 gene in the incompatible interaction, we silenced the gene in pepper plants using the tobacco rattle virus (TRV)-based VIGS technique with the full-length CaPO2 open reading frame (Liu et al., 2002; Chung et al., 2004). To assess the efficiency of VIGS, CaPO2 transcript levels were examined by reverse transcription (RT)-PCR in empty vector control (TRV:00) and CaPO2-silenced (TRV:CaPO2) pepper leaves 24 h after inoculation with Xcv (Fig. 4). CaPO2 transcripts were not detected upon virulent Xcv infection of the CaPO2-silenced leaves; however, they remained at a slightly visible level in the avirulent Xcv-infected leaves. To determine whether the expression of defense-related genes is affected by bacterial infection in the silenced plants, we further analyzed the transcript levels of several defense-related pepper genes by RT-PCR (Fig. 4). The CaBPR1, CaPR4 (putative antifungal protein), and CaPR10 genes were strongly induced by avirulent Xcv infection in the empty vector control plants (TRV:00); however, induction of CaBPR1 was compromised in CaPO2-silenced plants infected with either virulent or avirulent Xcv. Silencing of the CaPO2 gene also remarkably compromised the induction of the CaPOA1 (ascorbate peroxidase), CaDEF1 (defensin), CaOSM1 (osmotin-like protein), and CaSAR82 (SAR8.2) genes by Xcv infection, especially by the virulent strain.
Figure 4.
RT-PCR analysis of the expression of CaPO2 and pepper defense-related genes in empty vector control (TRV:00) and CaPO2 gene-silenced (TRV:CaPO2) pepper plants 24 h after inoculation with the virulent strain Ds1 (C, compatible) and the avirulent strain Bv5-4a (I, incompatible) of Xcv (5 × 106 cfu mL−1). The level of 18S rRNA was visualized as a control. This experiment was repeated three times with similar results. H, Healthy leaves.
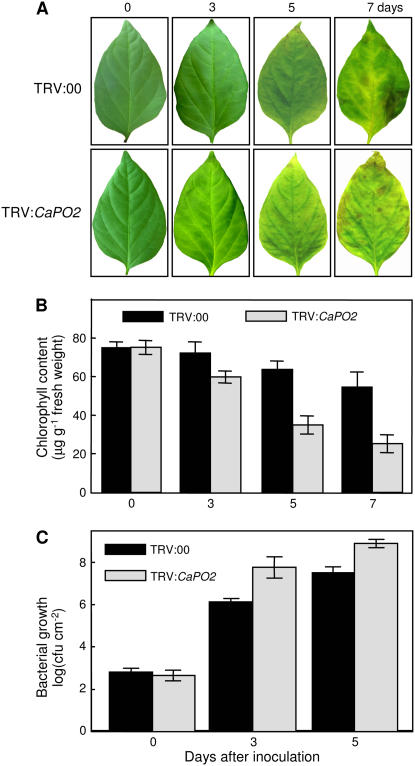
Silencing of the CaPO2 gene led to a highly susceptible response to infection by the Xcv virulent strain Ds1 (5 × 106 colony forming units [cfu] mL−1), accompanied by accelerated chlorosis (Fig. 5, A and B). CaPO2-silenced pepper leaves (TRV:CaPO2) started to exhibit chlorosis in periveinal regions 3 d after Xcv inoculation, whereas the empty vector control leaves (TRV:00) became chlorotic 5 d after inoculation. CaPO2-silenced leaves exhibited more severe chlorosis phenotypes than did the empty vector control leaves 7 d after inoculation. In particular, inoculation with the virulent strain Ds1 resulted in high levels of bacterial growth in the silenced plants compared with control plants (Fig. 5C). These data suggest that the extracellular peroxidase gene CaPO2 plays important roles in the basal resistance of pepper plants to virulent Xcv infection.
Figure 5.
Enhanced disease susceptibility of CaPO2 gene-silenced pepper plants to infection by the Xcv virulent strain Ds1. A, Representative disease symptoms developed on the leaves 0, 3, 5, and 7 d after inoculation (5 × 106 cfu mL−1). B, Chlorophyll contents of empty vector control (TRV:00) or CaPO2 gene-silenced (TRV:CaPO2) pepper plants 0, 3, 5, and 7 d after inoculation. C, Bacterial growth in leaves of the empty vector control (TRV:00) or CaPO2 gene-silenced (TRV:CaPO2) pepper plants 0, 3, and 5 d after inoculation (104 cfu mL−1). Data are the means ± sds from three independent experiments.
Silencing of the CaPO2 Gene in Pepper Compromises Oxidative Bursts and HRs, Locally and Systemically
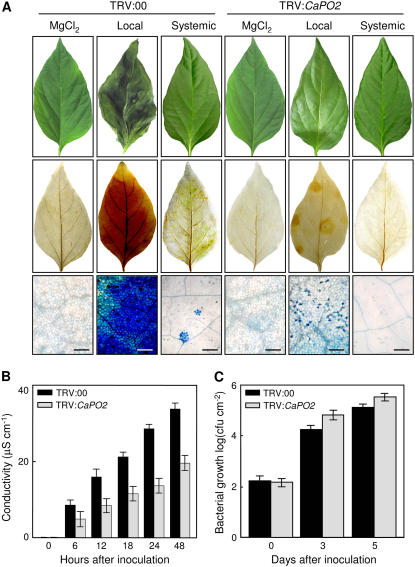
To investigate the roles of the CaPO2 gene in the oxidative burst and HR induction, H2O2 production and micro-HR formation in infected primary and uninfected secondary pepper leaves after inoculation with an avirulent Xcv strain were observed by staining with DAB and trypan blue. Silencing of the CaPO2 gene compromised not only oxidative bursts and the HR in local infected leaves but also reduced microbursts and micro-HR in uninoculated secondary leaves 24 h after infection with the Xcv avirulent strain Bv5-4a (Fig. 6A). This result suggests that extracellular peroxidase CaPO2-dependent ROS generation is required to generate oxidative bursts and induce the HR, both locally and systemically. Cell death was measured by ion leakage from leaf discs of empty vector control (TRV:00) and CaPO2-silenced (TRV:CaPO2) leaves inoculated with the avirulent strain Bv5-4a (Fig. 6B). The original conductivity strength in CaPO2-silenced pepper leaves was reduced by half compared with empty vector controls. In CaPO2-silenced leaves, small but significantly higher bacterial growth was observed 3 and 5 d after inoculation with the avirulent strain Bv5-4a as compared with TRV-infected control leaves (Fig. 6C), indicating that silencing of the CaPO2 gene enhanced susceptibility to infection by the avirulent strain Bv5-4a.
Figure 6.
Reduction of the oxidative burst and HR phenotypes in the CaPO2 gene-silenced pepper leaves infected by the Xcv avirulent stain Bv5-4a. A, Representative HR symptoms developed on the leaves 24 h after inoculation (5 × 106 cfu mL−1). Xcv-infected primary leaves were harvested and stained with DAB and trypan blue 1 and 24 h after inoculation, respectively; however, uninoculated secondary leaves were stained with both agents 24 h after inoculation. Bars = 200 μm. B, Cell death measured as ion leakage from leaf discs of empty vector control (TRV:00) and CaPO2 gene-silenced (TRV:CaPO2) pepper plants at various times after inoculation (5 × 106 cfu mL−1). Data are the means ± sds from three independent experiments. C, Bacterial growth in empty vector control (TRV:00) or CaPO2 gene-silenced (TRV:CaPO2) pepper leaves 0, 3, and 5 d after inoculation (104 cfu mL−1). Data are the means ± sds from three independent experiments.
Overexpression of CaPO2 in Arabidopsis Enhances Disease Resistance Accompanied by Micro-HRs
Because transformation is very difficult in pepper plants, we overexpressed CaPO2 in Arabidopsis to determine the in planta functions of the gene. To generate transgenic Arabidopsis plants expressing the CaPO2 gene, the CaPO2 open reading frame was integrated between the CaMV 35S promoter and the nos terminator region in the binary vector pBIN35S (Supplemental Fig. S2A). Arabidopsis ecotype Columbia (Col-0) plants were transformed using the 35S:CaPO2 construct according to the floral dipping method (Clough and Bent, 1998). Among 13 lines of CaPO2-OX plants, the CaPO2-OX mutant T2 lines 2, 11, and 13, which strongly expressed the CaPO2 gene, were selected for further experiments (Supplemental Fig. S2B). We did not observe any apparent phenotypic differences between wild-type and CaPO2-OX mutant lines. Wild-type and CaPO2-OX plants were evaluated for total peroxidase activity as described by Hammerschmidt et al. (1982; Supplemental Fig. S2C). Wild-type plants contained a low level of peroxidase activity, but all tested CaPO2-OX plants (T2) exhibited a 3- to 5-fold higher total peroxidase activity than wild-type plants. This result suggests that overexpression of the CaPO2 gene in Arabidopsis may enhance peroxidase activity.
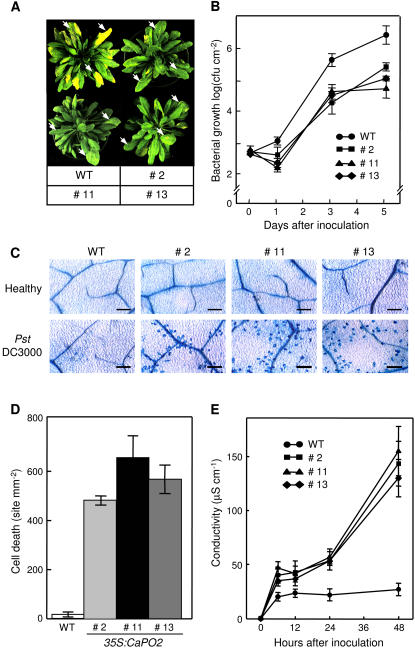
In the loss-of-function analysis, silencing of the CaPO2 gene increased susceptibility to Xcv infection, which suggested a possible role for CaPO2 in the defense response of pepper plants to bacterial pathogen infection (Figs. 5 and 6). For the gain-of-function analysis, we evaluated whether overexpression of the CaPO2 gene in Arabidopsis confers enhanced resistance to P. syringae pv tomato (Pst) DC3000 infection. Seven days after inoculation, disease symptoms developed on the leaves of wild-type plants but not on CaPO2-OX plants (Fig. 7A). All CaPO2-OX lines exhibited slightly decreased bacterial growth 1 d after inoculation, compared with wild-type plants. Three and 5 d after inoculation, however, all CaPO2-OX mutant plants examined exhibited an approximately 10-fold reduction in bacterial growth as compared to wild-type plants (Fig. 7B). Together, these findings suggest that overexpression of the CaPO2 gene enhances the basal resistance of Arabidopsis plants to Pst DC3000 infection.
Figure 7.
Enhanced resistance of Arabidopsis CaPO2-OX plants to Pst DC3000 infection accompanied by micro-HRs. A, Disease symptoms developed on the leaves of wild-type and CaPO2-OX plants 7 d after inoculation (104 cfu mL−1). Arrows indicate infected leaves. B, Bacterial growth in the leaves of wild-type and CaPO2-OX plants 0, 1, 3, and 5 d after inoculation (104 cfu mL−1). Data are the means ± sds from three independent experiments. C, Trypan blue staining of micro-HRs in leaf tissues of CaPO2-OX plants 24 h after inoculation (106 cfu mL−1). Bars = 200 μm. D, Quantification of cell death. Clusters of dead cells were identified and quantified by the trypan blue staining of wild-type and CaPO2-OX leaves 24 h after inoculation (106 cfu mL−1). Data are the means ± sds from three independent experiments. E, Ion leakage from leaf discs of wild-type and CaPO2-OX plants 0, 12, 24, 36, and 48 h after inoculation (106 cfu mL−1). Data are the means ± sds from three independent experiments.
Arabidopsis CaPO2-OX leaves infiltrated with 106 cfu mL−1 of Pst DC3000 (empty vector) exhibited numerous micro-HRs that left no detectable symptoms (Fig. 7, C and D). In contrast, a faint and negligible cell death response was observed in the leaves of wild-type plants following Pst DC3000 infection. Microscopic observations of trypan blue-stained leaves revealed that many cell death sites were located in periveinal regions in the leaves of CaPO2-OX plants (Fig. 7C). Consistent with the result of the trypan blue staining, CaPO2-OX plants exhibited significantly greater ion leakage levels compared with wild-type plants (Fig. 7E). Together, these results suggest that ectopic expression of CaPO2 in Arabidopsis increases basal resistance accompanied by a micro-HR response to virulent Pst DC3000 infection.
Overexpression of CaPO2 in Arabidopsis Increases H2O2 Generation and PR Gene Induction in Response to Virulent Pst DC3000 Infection
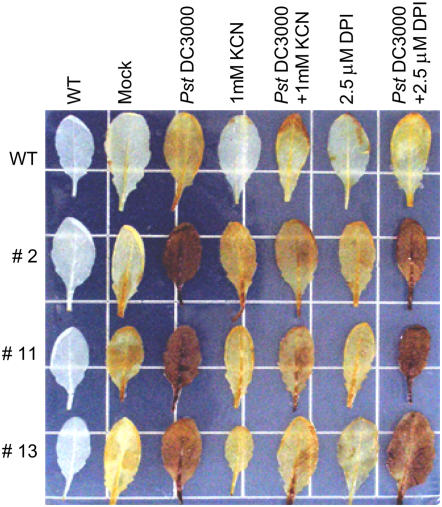
To determine whether the CaPO2 peroxidase acts as a ROS-detoxifying or -generating enzyme during pathogenesis, leaves of Arabidopsis wild-type and CaPO2-OX plants were stained with DAB after infiltration with Pst DC3000 (106 cfu mL−1). Wild-type leaves inoculated with Pst DC3000 were only faintly colored reddish brown by the DAB polymer. The leaves of Arabidopsis CaPO2-OX lines that constitutively expressed the transgene were stained a vivid reddish brown after inoculation with Pst DC3000 as compared with wild-type plants (Fig. 8). This result suggests that ectopic expression of CaPO2 in Arabidopsis induces H2O2 generation in response to Pst DC3000 infection. To determine whether the H2O2 generation is induced by the Pst DC3000 infection in a peroxidase-independent manner, we further quantified the H2O2 amounts using the xylenol orange assay (Gay et al., 1999; Bindschedler et al., 2001; Bindschedler et al., 2006; Supplemental Fig. S3). Consistent with the DAB staining results, CaPO2-OX induced higher levels of H2O2 in leaf tissues of all tested transgenic lines early after inoculation with Pst DC3000 compared to wild-type plants (Supplemental Fig. S3A).
Figure 8.
Detection of H2O2 in Arabidopsis wild-type and CaPO2-OX leaves by staining with DAB 10 min after infiltration with Pst DC3000 (empty vector; 106 cfu mL−1) in the presence or absence of 1 mm KCN (peroxidase inhibitor) or 2.5 μm DPI (NADPH oxidase complex inhibitor). WT, Wild type; Mock, mock treated.
To confirm whether the generation of H2O2 is induced by the CaPO2 protein, leaves of wild-type and CaPO2-OX plants were infiltrated with 106 cfu mL−1 of Pst DC3000 and supplemented with 1 mm KCN or 2.5 μm DPI (Fig. 8). In general, KCN and DPI inhibit peroxidase and NADPH oxidase activities, respectively (Pellinen et al., 1999; Orozco-Cárdenas et al., 2001; Zhang et al., 2001; Romero-Puertas et al., 2004). Coinfiltration of Pst DC3000 with 1 mm KCN effectively inhibited the CaPO2-mediated generation of H2O2 in leaves of CaPO2-OX plants, whereas treatment with 2.5 μm DPI did not. No changes in H2O2 accumulation were observed in wild-type pants infiltrated with 1 mm KCN or 2.5 μm DPI. Furthermore, similar results were observed by xylenol orange assay (Supplemental Fig. S3B). Coinfiltration of Pst DC3000 with 1 mm KCN, but not with 2.5 μm DPI, significantly lowered the H2O2 induction in leaves of CaPO2-OX line (no. 11). These findings suggest that H2O2 accumulation in CaPO2-OX plants after Pst DC3000 infection is CaPO2 peroxidase dependent but not NADPH oxidase independent.
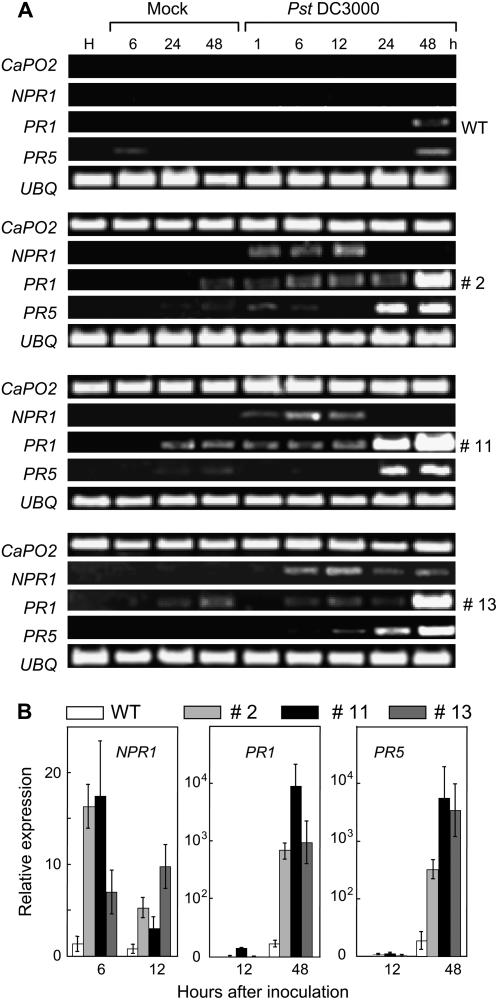
PR gene expression in Arabidopsis wild-type and CaPO2-OX plants was analyzed by RT-PCR and real-time RT-PCR (Fig. 9, A and B). The Arabidopsis PR genes examined were not expressed in healthy leaves of wild-type or CaPO2-OX plants. However, Pst DC3000 infection differentially induced the Arabidopsis PR genes NPR1, PR1, and PR5, which are known to be regulated by the salicylic acid (SA)-dependent pathway in CaPO2-OX plants compared with wild-type plants. In wild-type plants, PR1 and PR5 were slightly induced by mock and Pst DC3000 infection. However, CaPO2-OX plants exhibited a rapid and significantly enhanced induction of NPR1, PR1, and PR5 after mock and Pst DC3000 infection. The CaPO2-OX mutant line 11, which showed the highest expression level of CaPO2 and peroxidase activities, exhibited higher levels of induced NPR1, PR1, and PR5 than did the CaPO2-OX lines 2 and 13 (Fig. 9, A and B). This result suggests that ectopic expression of the CaPO2 gene in Arabidopsis triggers the SA-dependent defense pathway during virulent Pst DC3000 infection.
Figure 9.
Accelerated and increased expression of Arabidopsis PR genes in CaPO2-OX lines after infection with Pst DC3000. RT-PCR (A) and real-time RT-PCR (B) analyses of expression of the NPR1, PR1, and PR5 genes in Arabidopsis wild-type and CaPO2-OX plants at different times after inoculation (105 cfu mL−1). Expression of the UBQ gene served as a control. The data in B are the means ± sd from three independent experiments.
Overexpression of CaPO2 in Arabidopsis Accelerates Cell Death against Avirulent Pst DC3000 (avrRpm1 or avrRpt2) Infection
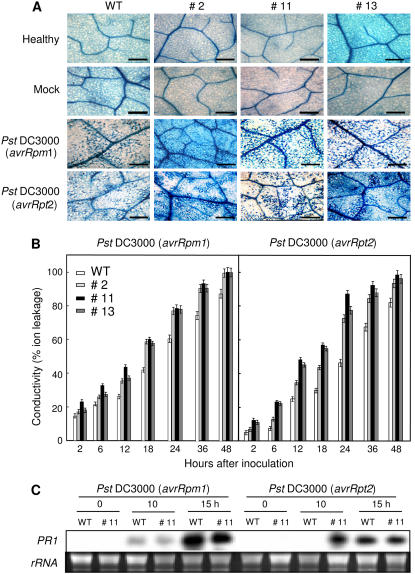
To determine whether CaPO2 overexpression in Arabidopsis influences the HR, wild-type and CaPO2-OX plants were infiltrated with avirulent Pst DC3000 carrying avrRpm1 or avrRpt2, followed by trypan blue staining and ion conductivity measurement. CaPO2-OX lines infiltrated with avirulent Pst DC3000 (avrRpm1 or avrRpt2) exhibited significantly accelerated and increased cell death phenotypes compared with wild-type plants (Fig. 10A). Consistent with the results of trypan blue staining, inoculation with avirulent Pst DC3000 (avrRpm1 or avrRpt2) resulted in a significant increase in ion conductivity levels in CaPO2-OX plants, as compared to wild-type plants (Fig. 10B).
Figure 10.
Accelerated HRs of Arabidopsis CaPO2-OX plants to Pst DC3000 carrying avrRpm1 or avrRpt2. A, Trypan blue staining to detect the HR in leaf tissues of wild-type and CaPO2-OX plants 24 h after inoculation (106 cfu mL−1). B, Cell death measured as ion leakage from leaf discs of wild-type and CaPO2-OX plants at various times after inoculation (106 cfu mL−1). Data are the means ± sds from three independent experiments. C, RNA gel-blot analysis of PR1 expression in wild-type and CaPO2-OX plants at various times after inoculation (106 cfu mL−1). The membrane was hybridized with a 32P-dCTP-labeled PR1 probe. Equal loading of total RNA (10 μg per lane) was verified by visualizing rRNA on a gel stained with ethidium bromide. WT, Wild type; #2, #11, and #13, Arabidopsis CaPO2-OX lines. Experiments were repeated three times with similar results.
PR1 induction by Pst DC3000 (avrRpm1 or avrRpt2) infection in wild-type and CaPO2-OX plants was monitored by RNA gel-blot analysis (Fig. 10C). There was no significant difference in PR1 expression between wild-type and CaPO2-OX plants 12 h after inoculation with Pst DC3000 carrying avrRpm1. However, PR1 was rapidly induced in CaPO2-OX plants challenged with avirulent Pst DC3000 (avrRpt2) compared to wild-type plants.
Overexpression of CaPO2 in Arabidopsis Enhances Systemic Microbursts and Micro-HRs against Pst DC3000 Infection
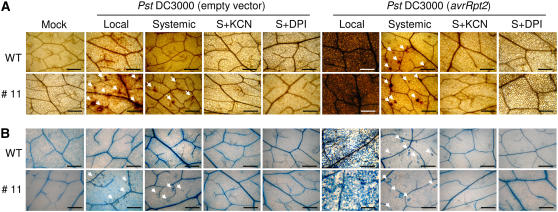
To determine whether overexpression of the CaPO2 gene increases microbursts and micro-HRs in uninoculated secondary leaves of Arabidopsis plants, primary and secondary leaves were stained with DAB and trypan blue 24 h after inoculation of primary leaves with Pst DC3000 (empty vector or avrRpt2; Fig. 11). Infection by avirulent Pst DC3000 (avrRpt2) resulted in strong oxidative bursts and hypersensitive cell death in inoculated primary leaves, as well as significant microbursts and micro-HRs in uninoculated secondary leaves. However, we did not observe microbursts or the HRs in the leaves of wild-type plants infected with virulent Pst DC3000. The induction of microbursts and micro-HRs was significantly greater in the secondary leaves of Arabidopsis CaPO2-OX plants compared with wild-type plants. The systemic microbursts and micro-HRs in wild-type and CaPO2-OX leaves were eliminated by infiltration of secondary leaves with KCN and DPI. These findings suggest that the ectopic expression of CaPO2 enhances the level of systemic microbursts and micro-HR foci in response to Pst DC3000 infection.
Figure 11.
Enhanced SAR phenotypes in Arabidopsis CaPO2-OX plants. DAB (A) and trypan blue (B) staining of primary and secondary leaves of wild-type and CaPO2-OX plants 24 h after challenging lower primary leaves with Pst DC3000 or Pst DC3000 (avrRpt2; 107 cfu mL−1). Secondary (S) leaves were also simultaneously infiltrated with 1 mm KCN (peroxidase inhibitor) or 2.5 μm DPI (NADPH oxidase complex inhibitor) immediately after Pst inoculation. Infected local leaves were stained with DAB and trypan blue 10 min and 24 h after inoculation, respectively. Arrows indicate microbursts and micro-HRs. Bars = 500 μm.
DISCUSSION
In a search for defense-related genes in a pepper cDNA library, we observed that the pepper extracellular peroxidase CaPO2 gene is locally or systemically induced by avirulent X. campestris pv. vesicatoria infection. In addition, staining with DAB and trypan blue revealed that avirulent Xcv infection strongly induces oxidative bursts and hypersensitive cell death, as well as CaPO2 expression, both locally and systemically. These findings allowed us to test whether the CaPO2 gene mediates oxidative bursts and cell death in both primary and secondary leaves of pepper plants in response to Xcv infection.
We used VIGS to dissect the biological functions of the CaPO2 gene in generating oxidative bursts and promoting cell death during the defense response in pepper plants. CaPO2-silenced plants were susceptible to virulent X. campestris pv. vesicatoria, which resulted in enhanced bacterial growth and accelerated chlorosis. Furthermore, VIGS of the CaPO2 gene also compromised oxidative burst generation and hypersensitive cell death during avirulent Xcv infection. These findings suggest that CaPO2-mediated ROS generation plays a pivotal role in basal resistance, as well as in the HR.
Multigene families of peroxidases are involved in various physiological processes in plants, and members of these families exhibit diverse expression patterns under stressful conditions (Hiraga et al., 2001; Passardi et al., 2004, 2005). These variable expression patterns are suggestive of pivotal roles for peroxidases in plant life cycles. Several peroxidase genes are expressed not only in response to pathogen attack but are also involved in the cross-linking of cell wall proteins or the generation of ROS. Growing numbers of publications have reported significant roles for peroxidases in defense responses (Hilaire et al., 2001; De Biasi et al., 2003; Bindschedler et al., 2006). Infection of rice leaves by Xanthomonas oryzae pv oryzae, the causal pathogen of rice blight, strongly induces a peroxidase isoform in xylem vessels, which results in secondary wall thickening and reduced access of the pathogen to the pit membrane, which is the pathogen's contact point in living cells (Hilaire et al., 2001). In tomato (Solanum lycopersicum) plants, H2O2 generation by cell wall peroxidase was demonstrated to be involved in the cell death response controlled by the Pto-Fen complex rather than by NADPH oxidases (De Biasi et al., 2003). More recently, studies with antisense FRENCH BEAN PEROXIDASE1 in transgenic Arabidopsis plants have suggested significant roles for peroxidases in the apoplastic oxidative burst and plant defense response (Bindschedler et al., 2006). However, the function of such extracellular peroxidases in hypersensitive cell death is not fully understood. In this study, we revealed biological functions for a novel pepper extracellular peroxidase gene—the CaPO2-dependent generation of ROS in hypersensitive cell death—by analysis of CaPO2-silenced pepper plants and CaPO2-OX transgenic Arabidopsis. Silencing of the CaPO2 gene not only enhanced susceptibility to Xcv infection but also compromised H2O2 accumulation and hypersensitive cell death in pepper leaves without inactivation of the NADPH oxidase complex in response to the Xcv avirulent strain Bv5-4a. Furthermore, strong H2O2 and micro-HR formation was detected in CaPO2-OX Arabidopsis plants after infection by a virulent strain of Pst DC3000, compared to wild-type Arabidopsis plants. These findings strongly support the notion that the CaPO2-dependent generation of ROS has a pivotal role in hypersensitive cell death.
The oxidative burst is essential for the establishment of plant immunity (Alvarez et al., 1998; Kim and Martin, 2004; Park, 2005). To determine whether CaPO2-mediated ROS accumulation and micro-HR formation are associated with the activation of PR gene expression, we carried out RT-PCR and real-time RT-PCR analyses in Arabidopsis. Strikingly, these analyses revealed that CaPO2-OX lines exhibited remarkably enhanced levels of PR1 and PR5 gene expression after inoculation with virulent Pst DC3000 (no avr gene). PR1 and PR5 genes are known as SA pathway-dependent defense-related genes. In particular, NPR1 (Arabidopsis nonexpressor of pathogenesis-related gene), a positive regulator of PR gene expression, was also detected in CaPO2-OX lines infected by virulent Pst DC3000. Furthermore, silencing of the CaPO2 gene significantly lowered CaBPR1 expression levels in pepper plants after Xcv infection. This finding suggests that CaPO2-mediated ROS accumulation stimulates PR gene expression. Silencing of CaPO2 also lowered the level of CaPOA1 gene expression after Xcv infection. Ascorbate peroxidase is known as a major ROS scavenging enzyme during the oxidative stresses (Muragia et al., 2004). The lowered levels of CaPOA1 transcript are consistent with the lowered oxidative burst levels in CaPO2-silenced pepper plants during pathogen infection. Therefore, we conclude that the CaPO2-mediated oxidative burst during pathogen infection triggers PR gene induction.
CaPO2-OX plants exhibited a significantly accelerated cell death response to infection by Pst DC3000 carrying avrRpm1 or avrRpt2. Moreover, PR1 induction was accelerated in Arabidopsis CaPO2-OX plants infected by Pst DC3000 carrying avrRpt2. The P. syringae type III effectors AvrRpm1 and AvrRpt2 have been demonstrated to posttranscriptionally phosphorylate and eliminate the RIN4 protein, thereby triggering RPM1 (resistance to P. syringae pv maculicola 1)- and RPS2-mediated defense signaling, respectively (Mackey et al., 2003). However, the downstream signaling and defense mechanism of RPM1 and RPS2 is not fully understood. Therefore, the relationship of the avrRpt2-specific accelerated cell death to PR1 gene expression in CaPO2-OX plants should be elucidated.
The generation of ROS (O2− and H2O2) is closely related to plant defense responses, especially the HR (Apel and Hirt, 2004; Wagner et al., 2004; Yun et al., 2006) and SAR (Alvarez et al., 1998). In earlier studies, the Arabidopsis NADPH oxidase was demonstrated to induce ROS during systemic resistance in the presence of an NADPH oxidase complex inhibitor (DPI) and catalase (Alvarez et al., 1998). However, the extracellular peroxidase-dependent generation of ROS during SAR has not yet been reported. To investigate whether pepper plants harbor NADPH oxidase complex- or peroxidase-dependent ROS generation systems in systemic microbursts and micro-HR foci, we used KCN and DPI as peroxidase and NADPH oxidase complex inhibitors, respectively. Treatment of secondary pepper leaves with both KCN and DPI eliminated microburst and micro-HR phenotypes induced by the Xcv avirulent strain Bv5-4a, accompanied by the elimination of CaPO2 gene expression. Moreover, silencing of the CaPO2 gene also compromised systemic microbursts and micro-HRs in pepper plants inoculated with the Xcv avirulent strain Bv5-4a. Interestingly, significant microbursts and micro-HRs were observed in the secondary leaves of CaPO2-OX plants but not of wild-type plants during virulent Pst DC3000 infection. These findings support the notion that the CaPO2 and NADPH oxidases are required for microbursts and micro-HRs in uninoculated secondary leaves of pepper plants. To the best of our knowledge, this is the first report noting the induction of the systemic microburst and micro-HR phenotype by extracellular peroxidase-dependent ROS generation. The induction of the oxidative burst and of defense-related genes such as CaPO2 in uninoculated secondary leaves may be essential for establishing SAR in pepper plants, as previously suggested (Lee and Hwang, 2005).
In human leukocytes activating immune response to pathogen infection, the heme-containing myeloperoxidase (MPO) is secreted at sites of infection and is involved in ROS generation and tissue damage (Arnhold, 2004). In particular, hereditary MPO deficiency leads to a recessive leukocyte disorder associated with defective candidacidal activity and increased susceptibility to pathogen infection. This observation suggests that MPO plays a significant role in the human defense system. Our primary concern was to determine how heme-containing pepper extracellular peroxidases CaPO2 contribute to defense responses of pepper plants to pathogen infection. Our results suggest pivotal roles for the extracellular peroxidase CaPO2 in oxidative burst generation, cell death, and PR gene induction for disease resistance in plants via the following mechanisms. First, recognition of pathogen invasion (especially by avirulent strains) induces CaPO2 gene expression in pepper plants. Second, CaPO2 protein is required for early H2O2 generation during pathogen infection. Arabidopsis CaPO2 transgenic plants that constitutively overexpress CaPO2 did not accumulate ROS in the absence of pathogen infection. However, significant ROS accumulation and micro-HR formation were detected during Pst DC3000 infection, especially avirulent Pst infection. Finally, CaPO2-dependent ROS generation activates defense responses, including hypersensitive cell death, PR gene induction, and SAR. The extracellular peroxidase CaPO2 and NADPH oxidase-mediated ROS generation is required for systemic microbursts and micro-HRs, which may contribute to the establishment of SAR. In principle, plants harbor two innate immune systems (Jones and Dangl, 2006). The first is a basal resistance which uses transmembrane pattern recognition receptors for the detection of slowly evolving pathogen-associated molecular patterns of pathogens. The second is a HR that uses R-protein-mediated recognition of avirulence proteins from avirulent pathogens. Taken together, our data suggest that CaPO2-mediated ROS generation is required for both basal resistance and HR in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Pepper (Capsicum annuum ‘Nockwang’) plants were grown in a plastic tray (55 × 35 × 15 cm) containing steam-sterilized soil mix (peat moss, perlite, and vermiculite; 5:3:2, v/v/v) and loam soil (1:1, v/v) at 28°C with a day length of 16 h at a light intensity of 70 μmol photons m−2s−1. Six seedlings at the two-leaf stage were transplanted to a plastic pot (5 × 15 × 10 cm) containing the same soil mix. Pepper plants at the six-leaf stage were used for pathogen infection and abiotic elicitor and environmental stress treatment.
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 plants were grown at 24°C with a photosynthetic flux of 130 μmol photons m−2 s−1 for 16 h (long day condition) or 12 h (short day condition) light and 60% relative humidity in a controlled environmental chamber. Plants were raised in pots containing vermiculite, peat moss, and perlite (1:1:0.5, v/v/v). Prior to sowing, the seeds of wild-type (Col-0) and CaPO2-OX plants were sterilized with a 2% sodium hypochlorite solution, followed by imbibition at 4°C for 3 d to overcome dormancy.
Pathogens and Inoculation Procedures
The virulent strain Ds1 and the avirulent strain Bv5-4a of Xcv were used in this study. Bacteria were cultured overnight in yeast-nutrient broth (5 g yeast [Saccharomyces cerevisiae] extract, 8 g nutrient broth L−1) at 28°C. Prior to inoculation, bacterial cells were collected by centrifugation and resuspended in 10 mm MgCl2 solution (108 cfu mL−1). Pepper plants at the six-leaf stage were inoculated by infiltrating the bacterial suspension into the abaxial side of fully expanded leaves using a syringe without a needle. The infected plants were incubated in a controlled chamber at 28°C with 100% relative humidity for 16 h. Infected primary and uninfected secondary upper leaves were harvested at various time points for bacterial growth assays, RNA isolation, and histochemical analysis.
Pst DC3000 was grown overnight in King's B medium containing 50 μg mL−1 rifampicin. To determine bacterial growth, leaves of 4-week-old wild-type and CaPO2 transgenic Arabidopsis T2 plants were infiltrated with 104 cfu mL−1 of Pst DC3000 in 10 mm MgCl2 using a syringe without a needle. Infected leaves were harvested 0, 1, 3, and 5 d after inoculation. Bacterial growth experiments were repeated three times with similar results.
Isolation and Sequence Analysis of Pathogen-Induced CaPO2 cDNAs
For construction of pathogen-induced cDNA library, the avirulent strain Bv5-4a of Xcv was used to inoculate pepper leaves. The pepper cDNA library was constructed using 5 μg poly(A)+ mRNA extracted from inoculated pepper leaves (Kim and Hwang, 2000). To isolate pathogen-inducible cDNAs from the pepper cDNA library, differential hybridization was performed according to the method of Jung and Hwang (2000). Among the cDNA clones tested, the CaPO2 cDNA clone hybridized strongly and differentially to the cDNA probes from leaves infected by the avirulent strain Bv5-4a of Xcv.
Particle Bombardment
The coding region of the CaPO2 gene was cloned between the CaMV 35 promoter and smGFP region of the binary vector p326GFP to generate a C-terminal fusion of smGFP to CaPO2. For particle bombardment, the plasmids were purified using QIAGEN plasmid maxi kits according to the manufacturer's instructions (Qiagen). Onion (Allium cepa) epidermis was bombarded with gold particles coated with plasmids using a Bio-Rad (Hercules) PDS-1000/He particle delivery system. Bombarded specimens were incubated for 24 h on 1× Murashige and Skoog agar media and observed using a MRC-1024 confocal laser-scanning microscope (Bio-Rad).
RNA Isolation and RNA Gel-Blot Analysis
Total RNA was extracted from pepper leaves, stems, roots, flowers, and fruits using the guanidine isothiocyanate method (Chomczynski and Sacchi, 1987). Total cellular RNA of CaPO2-OX Arabidopsis T2 plants was also extracted from the aerial portions of plants using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. To generate a gene-specific probe, the coding region of the CaPO2 gene was amplified using the primers CaPO2F (5′-ATGGCAGAGAAAACCACCAGCA-3′) and CaPO2R (5′-TCAAAAAAAAGTGACCTCCTTTCTGT-3′). The amplified PCR product was 32P labeled using a random priming kit (Boehringer Mannheim). Agarose gel electrophoresis, RNA transfers, and hybridization with the CaPO2 fragment were performed using standard procedures.
VIGS
The TRV-based VIGS system was used for gene silencing in pepper plants as described by Liu et al. (2002). The coding region of the pepper CaPO2 gene was cloned into the vector pTRV2 to yield pTRV2:CaPO2. Agrobacterium tumefaciens strain GV3101 carrying pTRV1 or pTRV2:CaPO2 was coinfiltrated into the fully expanded cotyledons of pepper plants (OD600 = 0.2). Plants were placed in a greenhouse at 25°C with a 16-h light and 8-h dark photoperiod cycle to allow growth and viral spread.
Arabidopsis Transformation
Transgenic Arabidopsis plants expressing the CaPO2 gene were generated using the floral dipping method (Clough and Bent, 1998). Thirteen lines of putative transgenic Arabidopsis plants (T1) harboring the 35S:CaPO2 construct were selected by planting seeds on Murashige and Skoog plates (Duchefa) containing 50 mg L−1 kanamycin. Three T2 lines, 2, 11, and 13, were used for further study.
Histochemistry
DAB staining was performed as described by Thordal-Christensen et al. (1997). Inoculated leaves were detached at various time intervals and placed in 1 mg mL−1 DAB (Sigma) solution for 15 h. To develop the redish-brown coloration of the DAB polymer, stained leaves were placed in boiling 95% ethanol for 10 min.
Trypan blue staining was performed as described by Koch and Slusarenko (1990). Infected leaves were sampled at the indicated time points after inoculation and stained with lactophenol-trypan blue solution (10 mL lactic acid, 10 mL glycerol, 10 g phenol, and 10 mg trypan blue, dissolved in 10 mL distilled water). Whole leaves were boiled for approximately 1 min in the staining solution and then decolorized in chloral hydrate (2.5 g chloral hydrate dissolved in 1 mL distilled water). They were mounted in 60% glycerol and representative phenotypes were photographed with a light microscope (Olympus, BH-2).
Real-Time RT-PCR and RT-PCR Analysis
The expression of the Arabidopsis NPR1, PR1, and PR5 genes during Pst DC3000 infection was examined using the real-time RT-PCR technique. Wild-type and CaPO2-OX Arabidopsis transgenic leaves were inoculated with 105 cfu mL−1 of Pst DC3000 and sampled 1, 6, 12, 24, and 48 h after inoculation. For mock treatment, 10 mm MgCl2 was used. Total RNA was extracted from the aerial portion of Arabidopsis T2 plants using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (2 μg), avian myeloblastosis virus reverse transcriptase (Roche), and oligo p(dT)15 primer (Roche) were used for cDNA synthesis. Gene-specific primer pairs for each amplification were: 5′-ATAGAGGACACATTGGTTATACTCAAGC-3′ and 5′-GATCGAGCAGCGTCATCTTCAATTCAT-3′ for NPR1, 5′-GTAGGTGCTCTTGTTCTTCCC-3′ and 5′-GTATGGCTTCTCGTTCACATAATTCC-3′ for PR1, 5′-TTCACATTCTCTTCCTCGTGTTCA-3′ and 5′-TCGTAGTTAGCTCCGGTACAAGTG-3′ for PR5, and 5′-CAAGACAGGAGAAATATGTCTCG-3′ and 5′-ATCCTTTCTTAGGCATAGCG-3′ for UBQ. For real-time RT-PCR analysis, the SYBR Green Supermix (Bio-Rad) was used according to the manufacturer's instructions.
Ion Leakage Measurement
Cell death in wild-type, CaPO2-silenced, and CaPO2-OX plants was assessed by ion leakage measurement after inoculation with Xcv (5 × 106 cfu mL−1) or Pst DC3000 (106 cfu mL−1). Six leaf discs of known area (0.6 cm in diameter) were removed and washed three times with distilled water following infiltration and incubated in 3 mL distilled water at room temperature. The conductivity of the incubation medium was recorded at various time points with a Crison conductivity meter (Net InterLab). The results from three independent experiments are represented as means ± sds.
Assay of Peroxidase Activity
To assess the total peroxidase activity, total proteins in wild-type and the CaPO2-OX plants were extracted by homogenizing the leaves with mortar and pestle added with 5 volumes of extraction buffer (0.1 m sodium phosphate buffer, pH 6.0, 0.5 m Suc). The homogenates were centrifuged for 20 min at 10,000g and 4°C. The clear supernatant was used for peroxidase activity assay according to the method of Hammerschmidt et al. (1982). Peroxidase activity was determined using guaiacol as the hydrogen donor. Leaf extract (100 μL) was added to the reaction mix (0.1 m sodium phosphate buffer, pH 6.0, 0.25% guaiacol, 1 m H2O2), followed by incubation for 5 min at 25°C. Peroxidase activity was determined by an increase in A470 min−1 mg−1 protein. Peroxidase activity of leaf extracts was calculated using the molar extinction coefficient of tetraguaiacol (2.66 × 104 mol−1 cm−1) and enzyme activity was expressed as nanokats per milligram of total proteins.
Quantification of H2O2 by Xylenol Orange Assay
H2O2 production in wild-type and CaPO2-OX Arabidopsis plants was monitored using xylenol orange assay (Gay et al., 1999; Bindschedler et al., 2001, 2006). The xylenol orange reagent was freshly prepared and remained stable for 6 to 8 h. One milliliter of solution [25 mm FeSO4 and 25 mm (NH4)2SO4, dissolved in 2.5 m H2SO4] was added to 100 mL of 125 μm xylenol orange and 100 mm sorbitol. Arabidopsis leaves were infiltrated with 107 cfu mL−1 of Pst DC3000 supplemented with either 1 mm KCN or 2.5 μm DPI. Fifteen leaf discs (0.28 cm2) excised using a cork borer were floated on 1 mL distilled water. To measure the H2O2 amount, 120 μL medium was withdrawn at various time points, briefly centrifuged for 15 s at 13,000g, and 100 μL of the supernatant was immediately added to 1 mL of xylenol orange reagent. The reaction mixture was incubated for 30 min at room temperature before measuring the A560 against the blank containing 100 μL distilled water. H2O2 production was expressed as relative values with 100% corresponding to the maximum value of the elicited samples.
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession numbers DQ489711 (CaPO2), AF053343 (CaBPR1), AF244122 (CaPR4), AF244121 (CaPR10), AF442387 (CaPOA1), AF442388 (CaDEF1), AY262059 (CaOSM1), AF313766 (CaSAR82), At1g64280 (NPR1), At2g14610 (PR1), At1g75040 (PR5), At2g29450 (GST1), and At4g05320 (UBQ).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Amino acid sequence alignment of pepper peroxidase CaPO2.
Supplemental Figure S2. Generation of the CaPO2-OX Arabidopsis mutants that expressed the CaPO2 gene.
Supplemental Figure S3. Quantification of H2O2 in leaves of wild-type (Col-0) and CaPO2-OX lines of Arabidopsis, using the xylenol orange assay.
Supplementary Material
Acknowledgments
We thank Dr. S.P. Dinesh-Kumar (Yale University) for the pTRV1 and pTRV2 vectors and Dr. U. Bonas (Martin-Luther-Universitaet) for Agrobacterium tumefaciens strain GV3101.
This work was supported by the Crop Functional Genomics Center of the 21st Century Frontier Research Program (grant no. CG1133) funded by the Ministry of Science and Technology, Korea; a grant from the Center for Plant Genetics and Breeding Research, Seoul National University, Korea; and by the Biogreen21 Program (grant no. 20070401034028), Rural Development Administration, Korea.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Byung Kook Hwang (bkhwang@korea.ac.kr).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92 773–784 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Arnhold J (2004) Free radicals-friends or foes? Properties, functions, and secretion of human myeloperoxidase. Biochemistry (Mosc) 69 4–9 [DOI] [PubMed] [Google Scholar]
- Babior BM (1984) The respiratory burst of phagocytes. J Clin Invest 73 599–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe DC (1999) Fast forward genetics based on virus-induced gene silencing. Curr Opin Plant Biol 2 109–113 [DOI] [PubMed] [Google Scholar]
- Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, et al (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschedler LV, Minibayeva F, Gardber SL, Gerrish C, Davies DR, Bolwell GP (2001) Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol 151 185–194 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F, Rowntree EG, Wojtaszek P (1999) Recent advances in understanding the origin of the cell wall oxidative burst in plant cells. Free Radic Res 31 S137–S145 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM (1998) Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol 116 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant defence—a broad perspective. Physiol Mol Plant Pathol 51 347–366 [Google Scholar]
- Bradley DJ, Kjellbon P, Lamb CJ (1992) Elicitor- and wound-induced oxidative cross-liking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70 21–30 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162 156–159 [DOI] [PubMed] [Google Scholar]
- Clough SH, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Chung E, Seong E, Kim YC, Chung EJ, Oh SK, Lee S, Park JM, Joung YH, Choi D (2004) A method of high frequency virus-induced gene silencing in chili pepper (Capsicum annuum L. cv. Bukang). Mol Cell 17 377–380 [PubMed] [Google Scholar]
- De Biasi MG, Astolfi S, Acampora A, Zuchi S, Fonzo V, Santangelo E, Caccia R, Badiani M, Soressi GP (2003) A H2O2-forming peroxidase rather than a NAD(P)H-dependent O2− synthase may be the major player in cell death responses controlled by the Pto-Fen complex following fenthion treatment. Funct Plant Biol 30 409–417 [DOI] [PubMed] [Google Scholar]
- Desikan R, Burnett EC, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide induce the expression of a homologue of gp91phox in Arabidopsis thaliana suspension cultures. J Exp Bot 49 1767–1771 [Google Scholar]
- Do HM, Hong JK, Jung HW, Kim SH, Ham JH, Hwang BK (2003) Expression of peroxidase-like genes, H2O2 production, and peroxidase activity during the hypersensitive response to Xanthomonas campestris pv. vesicatoria in Capsicum annuum. Mol Plant Microbe Interact 16 196–205 [DOI] [PubMed] [Google Scholar]
- Doke N (1983) Involvement of superoxide anion generation in hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal cell wall components. Physiol Plant Pathol 23 345–357 [Google Scholar]
- Gay C, Collins J, Gebicki JM (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273 149–155 [DOI] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J (2000) The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for oxidative burst and hypersensitive cell death. Plant J 23 441–450 [DOI] [PubMed] [Google Scholar]
- Groom OJ, Torres MA, Fordham-Skelton P, Hammond-Kosak KE, Robison NJ, Jones JDG (1996) RbohA, a rice homologue of mammalian pg91phox respiratory burst oxidase gene. Plant J 10 515–522 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R, Nuckles EM, Kuć J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20 73–82 [Google Scholar]
- Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire E, Young SA, Willard LH, McGee JD, Sweat T, Chittoor JM, Guikema JA, Leach JE (2001) Vascular defense responses in rice: peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol Plant Microbe Interact 14 1411–1419 [DOI] [PubMed] [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42 462–468 [DOI] [PubMed] [Google Scholar]
- Jabs T (1999) Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol 57 231–245 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- Jung HW, Hwang BK (2000) Isolation, partial sequencing, and expression of pathogenesis-related cDNA genes from pepper leaves infected by Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe Interact 13 136–142 [DOI] [PubMed] [Google Scholar]
- Kawano T (2003) Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 21 829–837 [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homologue of the neutrophil NADPH-oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hwang BK (2000) Pepper gene encoding a basic pathogenesis-related 1 protein is pathogen and ethylene inducible. Physiol Plant 108 51–60 [Google Scholar]
- Kim YJ, Martin GB (2004) Molecular mechanisms involved in bacterial speck disease resistance of tomato. Plant Pathol J 20 7–12 [Google Scholar]
- Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by downy mildew fungus. Plant Cell 2 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signaling: the latest news. Curr Opin Plant Biol 7 323–328 [DOI] [PubMed] [Google Scholar]
- Lamb CJ, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48 251–275 [DOI] [PubMed] [Google Scholar]
- Lee SC, Hwang BK (2005) Induction of some defense-related genes and oxidative burst is required for the establishment of systemic acquired resistance in Capsicum annuum. Planta 221 790–800 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon RA, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31 777–786 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balagué C, Roby D (2003) Lesion mimic mutants: key for deciphering cell death and defense pathways in plants? Trends Plant Sci 8 263–271 [DOI] [PubMed] [Google Scholar]
- Love AJ, Yun BW, Laval V, Loake GJ, Milner JL (2005) Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol 139 935–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112 379–389 [DOI] [PubMed] [Google Scholar]
- Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muragia I, Tarantino D, Vannini C, Bracale M, Carravieri S, Soave C (2004) Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 38 940–953 [DOI] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13 179–191 [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Doke N (2005) Convenient assay of O2− generated on potato tuber tissue slices treated with fungal elicitor by electron spin resonance—no secondary oxidative burst induction by H2O2 treatment. Plant Pathol J 21 283–287 [Google Scholar]
- Park JM (2005) The hypersensitive response, a cell death during disease resistance. Plant Pathol J 21 99–101 [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24 255–265 [DOI] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidase modify the cell wall. Trends Plant Sci 9 534–540 [DOI] [PubMed] [Google Scholar]
- Pellinen R, Palva T, Kangasjärvi J (1999) Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J 20 349–356 [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2− and H2O2 in pea leaves. Plant Cell Environ 27 1122–1134 [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C (1995) Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA 92 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11 1187–1194 [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues, AtrbohD and AtrobohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37 1130–1134 [DOI] [PubMed] [Google Scholar]
- Torres MA, Ouchi H, Hamada S, Machida C, Hammond-Kosak KE, Jones JDG (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14 365–370 [DOI] [PubMed] [Google Scholar]
- Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, et al (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306 1183–1185 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SC, Kim BS, Cha AR, Pack JP (2006) Ozone: changing anthracnose (caused by Colletotrichum acutatum) severity and accelerating hypersensitive response in pepper. Plant Pathol J 22 271–277 [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin A, Wojtaszek P, Bolwell GP (1994) Synthesis of dehydrogenation polymers of ferulic acid with high specificity by a purified cell-wall peroxidase from French bean (Phaseolus vulgaris L.). Biochem J 299 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.