Abstract
Fusing the TATA box-binding protein (TBP) to other DNA-binding domains may provide a powerful way of targeting TBP to particular promoters. To explore this possibility, a structure-based design strategy was used to construct a fusion protein, TBP/ZF, in which the three zinc fingers of Zif268 were linked to the COOH terminus of yeast TBP. Gel shift experiments revealed that this fusion protein formed an extraordinarily stable complex when bound to the appropriate composite DNA site (half-life up to 630 h). In vitro transcription experiments and transient cotransfection assays revealed that TBP/ZF could act as a site-specific repressor. Because the DNA-binding specificities of zinc finger domains can be systematically altered by phage display, it may be possible to target such TBP/zinc finger fusions to desired promoters and thus specifically regulate expression of endogenous genes.
TATA box-binding protein (TBP) plays a central role in eukaryotic transcription. The binding of TBP to a TATA box typically is the first step in assembly of the preinitiation complex (1) and, at many promoters, this is the rate-limiting step in transcriptional activation (2, 3). Many activators and repressors exert their effects through interactions with TBP or with proteins bound to TBP (4–6). TBP also interacts with other basal transcription factors such as TFIIA, -IIB, and -IIF, and with RNA polymerase II (7). The crystal structure of the TBP:DNA complex suggests how TBP can interact with so many other components of the transcriptional machinery (8, 9). The conserved COOH-terminal domain of TBP is essentially a saddle-shaped structure with approximate intramolecular two-fold symmetry. The concave surface of TBP contacts the minor groove of DNA, while the convex surface is exposed and available for interaction with other proteins. Sequence and structural comparisons show that the DNA-contacting residues (unlike the residues that are involved in protein–protein interactions) are almost perfectly conserved between the two pseudosymmetric subdomains. To initiate transcription by RNA polymerase II, TBP appears to bind to a TATA box in a specific orientation: the NH2-terminal subdomain of TBP contacts the 3′ (i.e., downstream) half of the TATA box, while the COOH-terminal subdomain contacts the 5′ (upstream) half of the TATA box (9, 10). Given the essentially symmetric arrangement of the DNA-contacting residues, it is not entirely clear how TBP distinguishes the proper orientation.
We have been interested in the structure-based design of novel transcription factors that can target particular genes, and we have extended the design methods developed by Pomerantz et al. (11) to create a fusion protein in which the three zinc fingers of Zif268 are connected to the COOH terminus of yeast TBP (yTBP). This fusion protein, designated TBP/ZF, has been used as a prototype for exploring how zinc fingers can target TBP to particular promoters and thus give site-specific regulation of gene expression.
MATERIALS AND METHODS
Protein Production and Purification.
A DNA fragment encoding TBP/ZF was generated by PCR and cloned in pET11a (Novagen). yTBP and TBP/ZF were produced as fusion proteins with His·Tag and purified by metal chelation affinity chromatography (Novagen). The DNA sequence after PCR and subcloning was confirmed. Protein concentration was measured with the protein assay kit (Bio-Rad).
Gel Shift Assay.
yTBP (5 μg/ml) or TBP/ZF (6 μg/ml) was incubated with labeled probe DNAs (0.1 nM) for 1 h at room temperature in 20 mM Hepes buffer (pH 8.0) containing 60 mM KCl, 5 mM MgCl2, 10% glycerol, 0.1% Nonidet P-40, 5 mM DTT, 0.1 mg/ml BSA, and 1 μg/ml salmon sperm DNA. Under these conditions, only a fraction (30–70%) of probe DNA was bound by protein. To begin measurement of dissociation rates, a large excess of unlabeled probe DNA (final concentration, 1 μM) was added to each incubation mixture at time t = 0. Aliquots were removed at several times and analyzed by gel electrophoresis.
In Vitro Transcription Analysis.
DNA fragments containing a promoter sequence and a G-free cassette cloned in pBluescriptIIKS+ (Stratagene) were isolated from an agarose gel after electrophoresis with QIAEX II kit (Qiagen, Chatsworth, CA). The DNA fragments were then biotinylated by Klenow extension, immobilized to M280 streptavidin dynabeads (Dynal, Great Neck, NY), and used as transcription templates. The transcription reaction was performed essentially as described (12).
Transient Cotransfection Assay.
Reporter plasmids were made by cloning five GAL4 binding sites and a promoter sequence in pGL3-Basic (Promega). The 293 cells were transfected by calcium phosphate precipitation with a glycerol shock as described (13). Luciferase activity was measured using luciferase assay reagent (Promega) and a ML2250 Luminometer (Dynatech) with the enhanced flash program and integration for 20 s with no delay.
RESULTS AND DISCUSSION
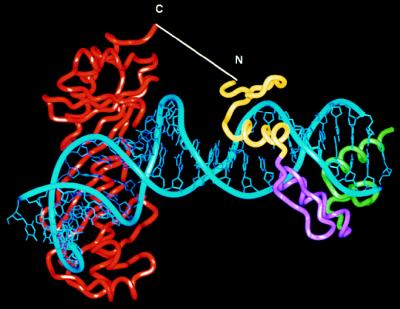
A model of the TBP/ZF:DNA complex is shown in Fig. 1. Preliminary modeling studies involved juxtaposing the Zif268:DNA (14) and the TBP:DNA complexes (9) in various orientations and registers to determine how the two polypeptide chains might be connected. As in Pomerantz et al. (11), the complexes were aligned by superimposing sets of phosphates, and modeling was facilitated by the fact that DNA outside of the TATA box is essentially canonical B-DNA (8, 9). A number of models were examined to find an arrangement that would (i) avoid steric interference between the domains and (ii) allow a relatively short connection between TBP binding in the minor groove and Zif268 binding in the major groove. The best model has a 4-bp spacer between the two binding sites and requires addition of a polypeptide linker that will span about 23 Å and thus connect the COOH terminus of TBP to the NH2 terminus of the three zinc finger Zif268 peptide. To proceed with biochemical studies, the two proteins were connected with a NH2-(Gly-Gly-Gly-Ser)2Gly-COOH linker, and it is expected that the fusion protein would bind tightly to a composite site with the sequence, 5′-GCGTGGGCGNNNNTATATAAA-3′.
Figure 1.
Structure-based design of TBP/ZF. The cocrystal structures of the Zif268:DNA (14) and the TBP:TATA box complexes (9) were aligned by superimposing phosphates in several different registers. In the model shown above, the NH2-terminal end of Zif268 was 23 Å away from the COOH-terminal end of TBP. We created the TBP/ZF fusion protein by adding a NH2-(Gly-Gly-Gly-Ser)2Gly-COOH polypeptide linker to join the two molecules. The alignment of binding sites used in this modeling study suggested that TBP/ZF would bind tightly to the sequence 5′-GCGTGGGCGNNNNTATATAAA-3′.
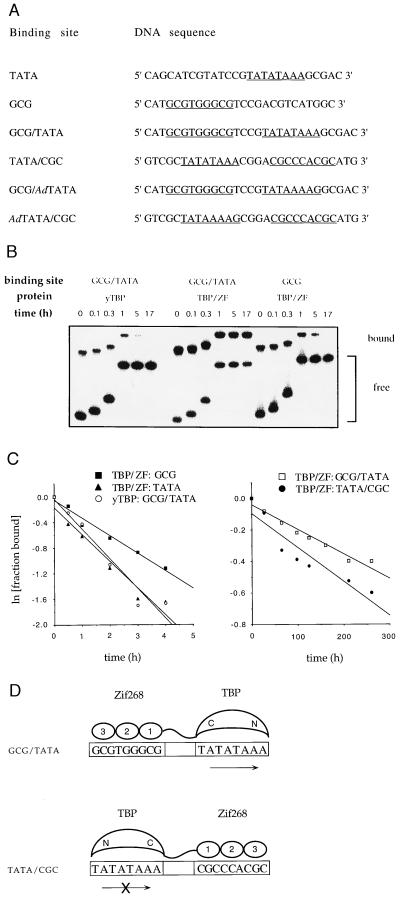
The TBP/ZF fusion protein was expressed in Escherichia coli, purified, and tested in gel shift assays (Fig. 2). TBP/ZF formed an extraordinarily stable complex (half-life, 410 h) with a DNA sequence (designated GCG/TATA) that had been chosen to match the site used in the computer modeling studies (Table 1). TBP/ZF also bound very tightly (half-life, 310 h) to the DNA sequence designated TATA/CGC. As discussed below and as shown in Fig. 2D, tight binding of TBP/ZF to the TATA/CGC site suggests that TBP can bind to the TATA box in an “inverted” configuration. The sequence of the TATA/CGC site (Fig. 2D Lower) may initially appear quite different from the sequence of the GCG/TATA site (Fig. 2D Upper), but these sites can readily be aligned by reading off of opposite strands. (Formally, this is equivalent to a two-fold rotation around an axis through the center of the TATA box.) Comparing sequences after aligning the sites shows that (i) the relative spacing of the Zif268- and TBP-binding sites is conserved and (ii) only two A/T differences are introduced when reading the other strand of this TATA box region. Thus, when the fusion protein binds to the TATA/CGC site, it can form a complex that is very similar to that shown in Fig. 1, but the TBP domain will be binding “backwards” with respect to the transcription start site (Fig. 2D Lower). Given the approximate two-fold symmetry of the TBP/TATA complex (8, 9), it seems quite plausible that TBP can be held in an “inverted” configuration when the fusion protein binds to the TATA/CGC site. (Modeling of the TATA/CGC complex shows that our linker is not long enough to allow both Zif and TBP to bind in the normal orientation without significant unfolding of the proteins or dramatic distortions in the DNA.) Binding studies also were performed with composite sites containing the TATA sequence from the adenovirus major late promoter (Fig. 2A). This TATA sequence is significantly less symmetric (rotating this sequence changes TATAAAAG to CTTTTATA). However, the fusion protein still formed very stable complexes with both of the composite sites: TBP/ZF had a half-life of 630 h on the GCG/AdTATA site and 220 h on the AdTATA/CGC site (Table 1). These results suggest that TBP can bind to the TATA box in either orientation and are consistent with the observation that the two pseudosymmetric subdomains of TBP have almost identical DNA-binding residues on their surfaces (8, 9).
Figure 2.
Determination of dissociation rate constants. (A) Probe DNA sequences used in gel shift assays. The TATA boxes and the Zif268-binding site are underlined. The sequence of only one strand is shown. (B) Example of gel shift assay used to determine dissociation rate constants. yTBP (5 μg/ml) or TBP/ZF (6 μg/ml) was incubated with labeled probe DNAs (0.1 nM) for 1 h at room temperature. To begin measurement of dissociation rates, a large excess of unlabeled probe DNA (final concentration, 1 μM) was added to each incubation mixture at time t = 0. Aliquots were removed at indicated times and analyzed by gel electrophoresis. Samples were loaded on the gel at different times, and thus the bands appear staggered. (C) The fraction of labeled probe DNA bound by protein was quantified by PhosphorImager (Molecular Dynamics) analysis, and normalized to the fraction bound at time t = 0. The natural log of the normalized fraction bound was plotted against time, and the dissociation rate was determined from the slope. (D) Models indicating how the orientationof the TBP moiety of TBP/ZF on the TATA box may be controlled by flanking Zif268-binding sites. The direction of transcription relative to the TATA box is shown with an arrow. (The “x” over the lower arrow indicates that this TBP orientation cannot support transcription.)
Table 1.
Dissociation rate constants for yTBP and TBP/ZF at various binding sites
| Protein | Binding site | koff, s−1 | t½, h |
|---|---|---|---|
| yTBP | GCG/TATA | 1.3 ± 0.1 × 10−4 | 1.5 |
| TBP/ZF | TATA | 1.2 ± 0.1 × 10−4 | 1.6 |
| TBP/ZF | GCG | 8.0 ± 1.4 × 10−5 | 2.4 |
| TBP/ZF | GCG/TATA | 4.7 ± 0.8 × 10−7 | 410 |
| TBP/ZF | TATA/CGC | 6.1 ± 0.1 × 10−7 | 310 |
| TBP/ZF | GCG/AdTATA | 3.0 ± 0.3 × 10−7 | 630 |
| TBP/ZF | AdTATA/CGC | 8.6 ± 1.0 × 10−7 | 220 |
The dissociation rate constants were determined in three separate experiments. The standard error of the mean is indicated.
Complexes of TBP/ZF with the individual binding sites were far less stable. The half-life of the TBP/ZF:TATA box complex (1.6 h) was almost identical with that of the yTBP:TATA-box complex (Table 1) (15, 16). It appears that the zinc finger domains of TBP/ZF do not affect the dissociation rate of the TBP moiety when the fusion protein is bound to the TATA box. Thus, the fusion protein binds over a hundred-fold more tightly when there are flanking zinc finger binding sites near the TATA box, and this should provide a suitable basis for differential promoter recognition.
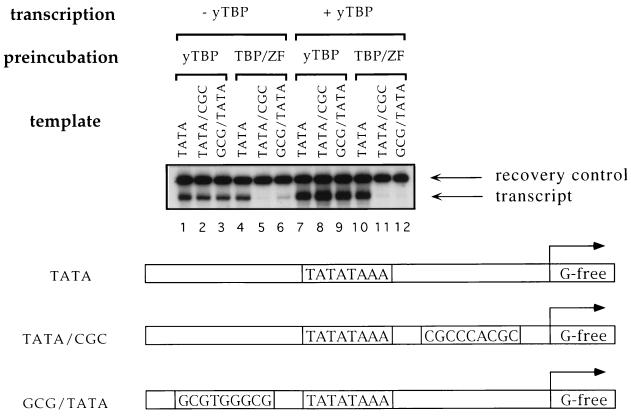
We next investigated the effect of TBP/ZF on in vitro transcription. Transcription templates were constructed to correspond to the three binding sites, TATA, TATA/CGC, and GCG/TATA used in the gel shift assays (Fig. 3). This in vitro transcription assay was performed in two steps to ensure that it measured the effects of fully active protein. First, TBP/ZF or yTBP was preincubated with each template. In a second step, the protein–template complex was isolated, and purified human transcription factors (TFIIB, -IIE, -IIF, and -IIH) and RNA polymerase II were added. Under these conditions, the fusion protein acted as a very effective repressor. TBP/ZF, when prebound to the TATA/CGC and GCG/TATA templates (Fig. 3, lanes 5 and 6), prevented the initiation of transcription, even if yTBP was added during the transcription reaction (lanes 11 and 12). Repression by the fusion protein required the presence of the adjacent zinc finger binding site: TBP/ZF allowed transcription from the TATA template (lanes 4 and 10) as effectively as yTBP did (lanes 1–3 and 7–9). These results indicate that the TBP moiety of the fusion protein can support transcription from the TATA template, since no TBP had been added in lane 4, and suggest that the added fingers do not interfere with transcription unless there is a specific binding site flanking the TATA box. Parallel tests with a peptide containing the three zinc fingers of Zif268 showed that it did not block transcription from any of these templates under these conditions (data not shown). Since the half-life of the Zif268:DNA complex is much shorter than that of the TBP/ZF complexes, it seems likely that the Zif268 peptide dissociated from the templates during the wash step (see Fig. 3 legend).
Figure 3.
In vitro transcription analysis. Biotinylated DNA fragments containing the promoters (TATA, TATA/CGC, and GCG/TATA) upstream of a G-free cassette were immobilized on streptavidin-coupled paramagnetic beads and used as transcription templates. yTBP at 6 μg/ml (lanes 1–3 and 7–9) or TBP/ZF at 8 μg/ml (lanes 4–6 and 10–12) was preincubated with each template (0.1 nM) for 1 h at room temperature. Then, supernatants were removed, and excess amounts (1 μM each) of competitor DNA oligonucleotides (GCG and TATA from Fig. 2A) were added to the preincubation mixture. After incubating 24 h at 4°C, the beads were washed to remove proteins that dissociated from the templates, and human transcription factors (TFIIB, -IIE, -IIF, and -IIH), RNA polymerase II, and substrate nucleotides were added to initiate transcription. yTBP was also added to a final concentration of 0.2 μg/ml in lanes 7–12. The transcripts were analyzed by urea gel electrophoresis.
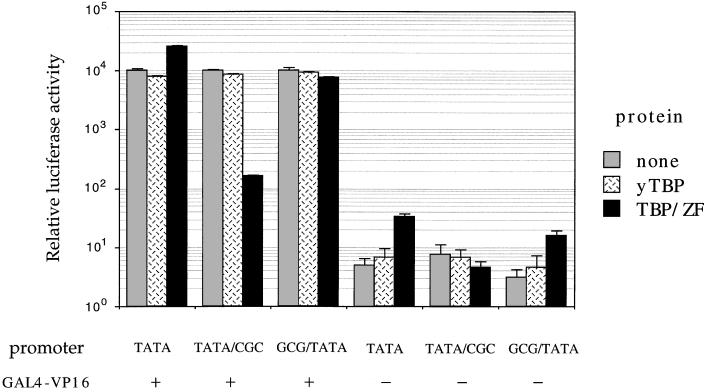
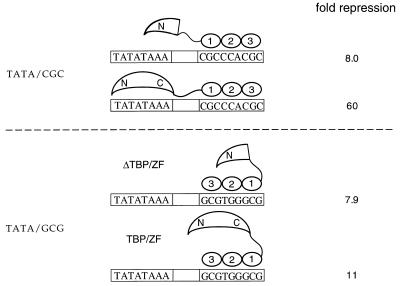
Transient cotransfection assays in the human cell line 293 were used to determine whether TBP/ZF could affect basal or VP16-activated transcription in vivo. In these experiments, expression plasmids encoding TBP/ZF or yTBP were cotransfected with reporter constructs encoding the firefly luciferase gene. yTBP showed no significant effect on basal or activated transcription at the TATA, GCG/TATA, and TATA/CGC promoters (Fig. 4). In contrast, TBP/ZF gave 60-fold repression of VP16-activated transcription from the TATA/CGC promoter. The fusion protein did not significantly affect activated transcription from the other two promoters. Binding of the fusion protein at the TATA/CGC site should have two distinctive features. First, when TBP/ZF binds to this site, the TBP domain will be facing “backwards” with respect to the transcription start site on this TATA box. [The linker is short enough that the COOH-terminal subdomain of TBP will now be on the “downstream” side of the TATA box (Figs. 1D and 5), and this may disrupt transcription.] Second, it seems likely that the zinc fingers themselves will occupy critical sites when bound to the TATA/CGC promoter and may help repress the initiation of transcription. [There are several examples of transcriptional repressors that act by binding in this region (17–19).]
Figure 4.
Transient cotransfection assay. Human 293 cells were cotransfected, using the calcium phosphate precipitation method with (i) 1 μg of expression plasmid encoding yTBP or TBP/ZF, (ii) 5 μg of activator plasmid, GAL4-VP16, (iii) 0.5 μg of β-galactosidase expression plasmid (pCMVβ) as an internal control, (iv) 1 μg of a reporter plasmid (derived from pGL3-Basic) encoding the firefly luciferase gene, and (v) variable amount of the carrier plasmid (pUC19) to keep the total amount of transfected DNA at 20 μg. Each reporter construct had five GAL4-binding sites upstream of one of the promoter sequences (TATA, TATA/CGC, or GCG/TATA) used in the in vitro transcription assay (Fig. 3). In a parallel assay of basal transcription, GAL4-VP16 was omitted. Luciferase activity was measured 2 days after transfection and was normalized (i) with respect to β-galactosidase activity (to correct for transfection efficiency), and (ii) to the corresponding value from the cells transfected with blank expression vector, pcDNA3 (which was set to an arbitrary value of 104). The absence or presence of GAL4-VP16 is indicated. The data represent an average of three independent experiments, and the standard error of the mean is shown.
Figure 5.
Transcriptional repression by TBP/ZF and ΔTBP/ZF in vivo. Transient cotransfection assays were used to determine whether TBP/ZF and ΔTBP/ZF could affect VP16-activated transcription from the TATA/GCG promoter. The results were compared with those from the TATA/CGC promoter (Fig. 4). The data represent an average of three independent experiments.
Control experiments indicate that simply occupying the Zif268 site is partially responsible for repression at the TATA/CGC promoter. In particular, we found that a TBP/ZF mutant in which residues 126–237 of the TBP moiety had been deleted gave an 8-fold repression of activated transcription from this promoter (Fig. 5). This is significant but also is lower than the 60-fold repression obtained with TBP/ZF. Immunoblot analysis showed that TBP/ZF and the deletion mutant were present at comparable levels in the nucleus (data not shown). We also tested the fusion protein at an alternative promoter that, like TATA/CGC, had the Zif268 site 4 bp downstream of the TATA box but, unlike TATA/CGC, had the Zif268 site oriented so that the TBP domain of the fusion protein could not reach the TATA box (Fig. 5). (By analogy with the nomenclature in Fig. 2A, this site was designated TATA/GCG.) At this site, TBP/ZF and the deletion mutant showed comparable levels of repression (11-fold and 8-fold, respectively). These results also indicate that simply occupying the Zif268 site is partially responsible for the 60-fold repression we had observed at the TATA/CGC site with TBP/ZF, but that the TBP domain must bind to the TATA box for the full effect.
The crystal structure of the TFIIB:TBP:TATA box complex (20) provides a clear explanation for repression at the GCG/TATA site in the in vitro transcription assays. TFIIB contacts a number of base pairs on the upstream side of the TATA box, and comparing the model in Fig. 1 with the crystal structure of the ternary complex shows that TBP/ZF would block normal binding of TFIIB at promoters containing the GCG/TATA site. In contrast to the in vitro transcription assays, TBP/ZF did not repress transcription from the GCG/TATA promoter in vivo. Given the large number of cellular factors that interact with a promoter region and with the transcription complex, it seems plausible that one or more of these factors (such as TFIIA) could disrupt or prevent binding of the zinc fingers at the GCG/TATA promoter in vivo and thus prevent TBP/ZF from acting as a repressor at this promoter in vivo.
It is interesting to compare our results with previous studies that have linked TBP to other DNA-binding proteins and have generated transcriptional activators (21–23). These studies have provided useful information about the mechanism of transcriptional activation, but our approach has a number of distinctive features. First, our strategy for constructing TBP/ZF was based on detailed computer modeling, and using a relatively short linker made it possible to control the orientation of TBP on the TATA box. Second, initial studies have shown that TBP/ZF, unlike previous TBP fusions, can act as a site-specific repressor. (We assume that these different effects on transcription reflect the very different spacing and arrangement of binding sites used with these different fusion proteins. It seems possible that we could create transcriptional activators by changing the spacing between the TATA box and the Zif268-binding site and adjusting the length of the linker between TBP and the zinc finger peptide.) Finally, it should be possible to redesign TBP/ZF to target specific endogenous promoters. Zinc fingers with novel DNA-binding specificities can be selected by phage display systems (24–27), and recent results suggest that it may be possible to select zinc finger proteins that will specifically recognize almost any desired site (28). By targeting zinc fingers to the flanking bases and constructing appropriate TBP/ZF variants, it may be possible to specifically recognize any desired promoter and thus regulate endogenous gene expression. Given the extraordinary stability of the TBP/ZF:DNA complexes, they may also provide a basis for careful physical and structural studies directed toward understanding how TBP interacts with noncanonical binding sites.
Acknowledgments
We thank D. I. Chasman and S. T. Smale for providing plasmids, S. Buratowski for a rabbit antibody against yTBP, S. K. Burley and D. B. Nikolov for TFIIB:TBP:TATA box coordinates, L. Nekludova for help with graphics, and J. L. Pomerantz for helpful discussions and critical reading of the manuscript. K.L.C. is supported by the Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation Fellowship. This work was supported by the National Institutes of Health and the Howard Hughes Medical Institute.
ABBREVIATIONS
- TBP
TATA box-binding protein
- yTBP
yeast TBP
References
- 1.Buratowski S, Hahn S, Guarente L, Sharp P A. Cell. 1989;56:549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- 2.Klein C, Struhl K. Science. 1994;266:280–282. doi: 10.1126/science.7939664. [DOI] [PubMed] [Google Scholar]
- 3.Colgan J, Manley J L. Genes Dev. 1992;6:304–315. doi: 10.1101/gad.6.2.304. [DOI] [PubMed] [Google Scholar]
- 4.Stringer K F, Ingles C J, Greenblatt J. Nature (London) 1990;345:783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- 5.Um M, Li C, Manley J L. Mol Cell Biol. 1995;15:5007–5016. doi: 10.1128/mcb.15.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J-L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Sun X, Reinberg D, Ebright R H. Proc Natl Acad Sci USA. 1996;93:1119–1124. doi: 10.1073/pnas.93.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y, Geiger J H, Hahn S, Sigler P B. Nature (London) 1993;365:512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- 9.Kim J L, Nikolov D B, Burley S K. Nature (London) 1993;365:520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- 10.Strubin M, Struhl K. Cell. 1992;68:721–730. doi: 10.1016/0092-8674(92)90147-5. [DOI] [PubMed] [Google Scholar]
- 11.Pomerantz J L, Sharp P A, Pabo C O. Science. 1995;267:93–96. doi: 10.1126/science.7809612. [DOI] [PubMed] [Google Scholar]
- 12.Parvin J D, Shykind B M, Meyers R E, Kim J, Sharp P A. J Biol Chem. 1994;269:18414–18421. [PubMed] [Google Scholar]
- 13.Cepek K L, Chasman D I, Sharp P A. Genes Dev. 1996;10:2079–2088. doi: 10.1101/gad.10.16.2079. [DOI] [PubMed] [Google Scholar]
- 14.Pavletich N P, Pabo C O. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 15.Hoopes B C, LeBlanc J F, Hawley D K. J Biol Chem. 1992;267:11539–11547. [PubMed] [Google Scholar]
- 16.Kuddus R, Schmidt M C. Nucleic Acids Res. 1993;21:1789–1796. doi: 10.1093/nar/21.8.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Hermiston T W, Stinski M F. J Virol. 1991;65:897–903. doi: 10.1128/jvi.65.2.897-903.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond I A, Madden S L, Rohwer-Nutter P, Bell G I, Sukhatme V P, Rauscher F J., III Science. 1992;257:674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- 19.Lee G, Wu J, Luu P, Ghazal P, Flores O. Proc Natl Acad Sci USA. 1996;93:2570–2575. doi: 10.1073/pnas.93.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolov D B, Chen H, Halay E D, Usheva A A, Hisatake K, Lee D K, Roeder R G, Burley S K. Nature (London) 1995;377:119–128. doi: 10.1038/377119a0. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee S, Struhl K. Nature (London) 1995;374:820–822. doi: 10.1038/374820a0. [DOI] [PubMed] [Google Scholar]
- 22.Klages N, Strubin M. Nature (London) 1995;374:822–823. doi: 10.1038/374822a0. [DOI] [PubMed] [Google Scholar]
- 23.Xiao H, Friesen J D, Lis J T. Mol Cell Biol. 1995;15:5757–5761. doi: 10.1128/mcb.15.10.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebar E J, Pabo C O. Science. 1994;263:671–673. doi: 10.1126/science.8303274. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Yang W-P, Barbas C F., III Proc Natl Acad Sci USA. 1995;92:344–348. doi: 10.1073/pnas.92.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamieson A C, Kim S-H, Wells J A. Biochemistry. 1994;33:5689–5695. doi: 10.1021/bi00185a004. [DOI] [PubMed] [Google Scholar]
- 27.Choo Y, Klug A. Proc Natl Acad Sci USA. 1994;91:11163–11167. doi: 10.1073/pnas.91.23.11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greisman H A, Pabo C O. Science. 1997;275:657–661. doi: 10.1126/science.275.5300.657. [DOI] [PubMed] [Google Scholar]