Abstract
The interleukin-2 (IL-2) receptor (IL-2R) is composed of three subunits. Of these, IL-2Rα is required for high-affinity IL-2 binding, while IL-2Rβ and IL-2Rγc are required for the transduction of IL-2-generated signals. Signals transduced via the S region of the IL-2Rβ (amino acids 267–322) in BAF/3 cells activate the phosphatidylinositol 3-kinase (PI3-kinase) and induce the expression of Bcl-2 and c-myc. Through the induction of Bcl-2, IL-2 inhibits apoptosis and through the combination of Bcl-2 and c-myc it stimulates progression through the cell cycle. Here we show that the protein kinase encoded by the Akt proto-oncogene is activated by IL-2. Akt activation by IL-2 depends on PI3-kinase signals transduced via the S region of the IL-2Rβ and is linked to the translocation of Akt to the cell membrane. Expression of catalytically active Akt mutants in BAF/3 cells expressing IL-2Rβ[A0]ΔS promotes the expression of Bcl-2 and c-myc, inhibits apoptosis induced by IL-3 deprivation or staurosporine, and stimulates cell cycle progression. The same mutants also stimulate cell cycle progression in 2780a, an IL-2-dependent T cell line that undergoes G1 arrest rather than apoptosis after IL-2 deprivation. The activation of Akt by IL-2 via the PI3-kinase and the rescue of the PI3-kinase-mediated antiapoptotic and proliferative IL-2 signals by catalytically active Akt indicate that these signals are transduced by Akt.
The interleukin (IL)-2 receptor (IL-2R) is composed of three subunits α, β, and γc. Of these, IL-2Rα is required for high-affinity IL-2 binding, while IL-2Rβ and IL-2Rγc are required for the transduction of IL-2-generated signals (1, 2). IL-2Rβ is shared by the receptors of at least two different cytokines (IL-2 and IL-15) (1–3), while IL-2Rγc is shared by the receptors of IL-2, IL-4, IL-7, IL-9, and IL-15 (4). IL-2Rβ and IL-2Rγc transduce signals via kinases and perhaps other molecules they bind to. Among those particularly important are the cytoplasmic tyrosine kinases Lck and Syk, which bind to IL-2Rβ (5, 6), the Janus kinases JAK1 and JAK3, which bind to the “serine rich” region of IL-2Rβ, and the C-terminal domain of the IL-2Rγc, respectively (7, 8), and the adaptor protein Shc (9). Recent studies showed that BAF/3 cells transfected with IL-2Rβ express the complete IL-2R complex and respond mitogenically to IL-2 stimulation. Stimulation of these cells with IL-2 activates at least three distinct pathways leading to the induction of Bcl-2, c-myc, and Lck. Cooperation between any two of these pathways appears to be sufficient for mitogenesis (10). The induction of Bcl-2 and c-myc depends on signals transduced from the S region of the IL-2Rβ (10). Signals originating from the same region of the receptor activate the phosphatidylinositol 3-kinase (PI3-kinase) (11). However, it is not known to date whether the activation of the PI3-kinase is responsible for the induction of Bcl-2 and c-myc.
The serine-threonine protein kinase encoded by the Akt proto-oncogene is activated by a variety of growth factors and intracellular signaling molecules via signals transduced by the PI3-kinase (12–19). Because both the activation of the PI3-kinase and the induction of Bcl-2 and c-myc depend on signals originating in the S region of the IL-2Rβ (10, 11), we questioned whether Akt is activated by IL-2 and whether, after activation, it induces expression of Bcl-2 and c-myc.
The data presented in this report show that Akt is activated by IL-2. Akt activation by IL-2 depends on PI3-K-mediated signals originating in the S region of the IL-2Rβ and is linked to its translocation to the cell membrane. Expression of catalytically active Akt mutants in BAF/3 cells expressing the wild-type IL-2Rβ, but not its ΔS (amino acids 267–322) mutant, promotes the expression of Bcl-2 and c-myc, inhibits apoptosis induced by growth factor deprivation or staurosporine, and stimulates cell cycle progression. The same mutants also stimulate cell cycle progression in 2780a, an IL-2-dependent T cell line, which undergoes G1 arrest rather than apoptosis after IL-2 withdrawal. These data indicate that the IL-2 antiapoptotic and proliferative signals that induce Bcl-2 and c-myc originate in the S region of the IL-2Rβ and are transduced via the PI3-kinase/Akt pathway.
MATERIALS AND METHODS
Cell Lines and Culture Conditions.
EL4·IL-2 (ATTC TIB 181) cells were purchased from the American Type Culture Collection, and BAF/3 cells were kindly provided by G. A. Evans (National Cancer Institute, Frederick, MD). 2780a is an IL-2-dependent rat T cell lymphoma line, which was established in this laboratory and undergoes G1 arrest rather than apoptosis after IL-2 withdrawal (20, 21). EL4·IL-2 cells were cultured at 37°C and 5% CO2 in DMEM supplemented with 10% (vol/vol) horse serum and penicillin (50 units/ml), streptomycin (50 μg/ml), and kanamycin (100 μg/ml) (PSK). BAF/3 and 2780a cells were cultured at 37°C and 5% CO2 in RPMI medium 1640 supplemented with 10% fetal bovine serum, PSK, and 10% WEHI cell supernatant (IL-3 source), or IL-2 (100 units per ml), respectively.
Expression Constructs and Transfections.
Expression constructs of IL-2Rβ (pdKCR-IL-2Rβ) and the IL-2Rβ·ΔS mutant (pLCKRβ-S) (10) were kindly provided by Zhao-Jun Liu and T. Taniguchi (University of Tokyo, Tokyo, Japan). Wild-type Akt and a myristylated Akt mutant, fused to a C-terminal hemagglutinin epitope tag (Akt·HA and Myr-Akt·HA), were cloned in the cytomegalovirus (CMV)-based expression vector CMV-6 and the retrovirus vector SRα. Finally, a PH domain Akt mutant (E40K) that exhibits enhanced basal kinase activity, but responds to physiological stimuli, (A.B., unpublished work) was cloned in CMV-6. BAF/3 cells were transfected by electroporation with the IL-2Rβ or IL-2Rβ·ΔS constructs, and they were selected for hygromycin resistance. The cells selected after transfection of these constructs were supertransfected also by electroporation with CMV-6 or the CMV-6-based expression constructs of Akt along with a plasmid encoding the Neor gene, and they were selected for G418 resistance. Finally, 2780a cells were infected with SRα or the SRα-based myristylated Akt constructs.
Transient transfections of Akt·HA and Myr-Akt·HA in EL4 cells were carried out by electroporation as previously described (21).
In Vitro Kinase Assays.
Five × 106 EL4·IL-2 cells or BAF/3 cells stably transfected with the IL-2Rβ or the IL-2Rβ·ΔS mutant were cultured at a concentration of 0.5 × 106 cells per ml. Subsequently, the EL4·IL-2 cells were serum-starved, and the BAF/3 cells were IL-3- and IL-2-starved for 16 h. Some of the starved cultures then were stimulated with IL-2 (100 units/ ml) for 10 min. Cells were lysed using an Nonidet P-40 lysis buffer (1% Nonidet P-40/10% glycerol/137 mM NaCl/20 mM Tris·HCl, pH 7.4) containing 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM sodium pyrophosphate, and 1 mM Na3VO4. Akt was immunoprecipitated from the cell lysates with the anti-Akt-CT antibody (12, 17, 22). Kinase assays were performed as described previously using histone H2B as the exogenous substrate (12, 17, 22). The products of the in vitro kinase reaction were analyzed by SDS/PAGE. The same protocol also was used to carry out in vitro kinase assays of Akt[A0]HA and Myr-Akt·HA immunoprecipitated with the anti-HA monoclonal antibody 12CA5 from transiently transfected EL4·IL-2 cells.
Immunofluorescence.
107 EL4·IL-2 cells were transiently transfected with C-terminally tagged Akt (Akt·HA) or myristylated Akt (Myr-Akt·HA) expression constructs and were cultured at the concentration of 0.5 × 106 cells per ml. Twenty-four hours after transfection the cells were serum-starved for 16 h, and then were stimulated with IL-2 (100 units/ml). Cells were harvested before, and 10 min after, stimulation with IL-2 and were stained with the anti-hemagglutinin monoclonal antibody 12CA5 and an anti-mouse fluorescein isthiocyanate-conjugated secondary antibody (Sigma). Immunofluorescence staining was carried out as follows. 105 cells were resuspended in 100 μl of complete medium and were evenly spread on microscope slides using a cytocentrifuge. The cells then were fixed in 3.5% fresh paraformaldehyde for 15 min and washed three times with PBS plus 0.1% Tween 20 (wash solution). Subsequently, they were incubated with the primary antibody 12CA5 (1:250 dilution) at room temperature for 1 h and then washed three times with wash solution before incubation for 45 min with the secondary antibody (1:100 dilution). After three additional washes, cell nuclei were stained with Hoechst 33258 (0.5 μg/ml) for 1 min and were washed three more additional times. Coverslips were mounted on the microscope slides with Vectorshield (Vector Laboratories).
Cell Death and Cell Cycle Analyses.
BAF/3-IL-2Rβ·ΔS cells, stably transfected with a CMV-6 construct of the carboxyl-terminally tagged myristylated Akt (Myr·Akt·HA) or the CMV-6 vector alone, were cultured in IL-2- and IL-3-deficient media at the concentration of 0.5 × 106 cells per ml. Live and dead cells were counted daily by trypan blue exclusion. The numbers of live and dead cells then were used to calculate the ratio of dead/dead + live cells. Based on this ratio we calculated the mean and the SD of the percentage of dead cells at sequential time points after growth factor withdrawal. The same methods were used to calculate the percentage of dead cells in BAF/3 cells expressing IL-2R·ΔS with or without myristylated Akt and treated with staurosporine (2 μM).
BAF/3 cell lines expressing IL-2Rβ·ΔS and myristylated Akt (BAF/3·ΔS·MA1 and MA2) and 2780a T cells infected with SRα or SRα-based myristylated Akt constructs were incubated with ethidium bromide in FACS buffer (3.4 mM sodium citrate/10 mM NaCl/0.1% Nonidet P-40/75 μM ethidium bromide) at the 72-h (3 days) time point after IL-3 withdrawal, and they were analyzed for DNA content by flow cytometry as described (23).
Western Blotting.
106 cells (BAF/3, EL4·IL-2 or 2780a) transfected transiently or stably with a variety of expression constructs were lysed after serum- and growth-factor starvation and treatment with various growth factors as described in Results. Lysis was carried out using the Nonidet P-40 lysis buffer described earlier. SDS/PAGE of the cell lysates or immunoprecipitates were probed with the following antibodies: anti-Akt·CT (12) or anti-HA (12), anti-Bcl-2 (Santa Cruz Biotechnology), and anti-c-myc (Upstate Biotechnology, Lake Placid, NY). Western blots were developed using enhanced chemiluminescence (Amersham) as previously described (12).
RESULTS
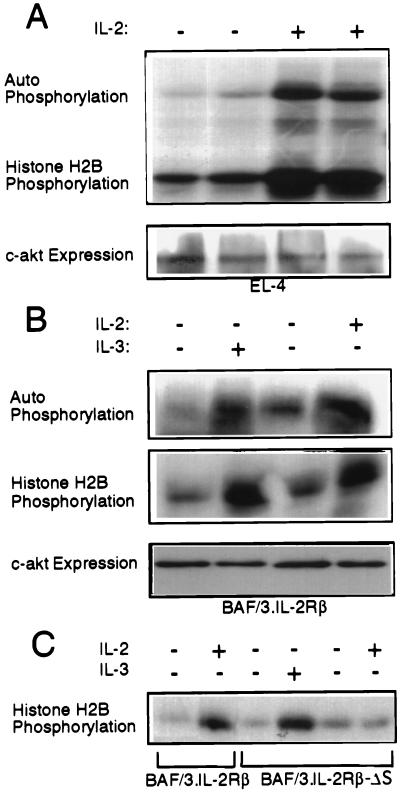
To determine the involvement of Akt in the transduction of IL-2 antiapoptotic and proliferative signals we first examined whether IL-2 activates Akt in serum-starved EL4[A0]IL-2 cells and in IL-2- and IL-3-starved BAF/3 cells engineered to stably express the IL-2Rβ (3). The results (Fig. 1A and B) showed that Akt is indeed activated by IL-2 as well as by IL-3. Akt activation by IL-2 and IL-3 similarly to its activation by other growth factors (6–9) was inhibited by wortmannin (6), a PI3-kinase inhibitor, and Δp85α, a dominant negative mutant of the PI3-kinase (24) (data not shown). In addition, IL-2, but not IL-3, failed to activate Akt in BAF/3 cells expressing an S region deletion mutant of the IL-2Rβ (ΔS), which does not activate the PI3-kinase in response to IL-2 stimulation (11) (Fig. 1C).
Figure 1.
Akt is activated by IL-2 and IL-3 in EL4·IL-2 cells and BAF/3 cells expressing IL-2Rβ, but not IL-2Rβ·ΔS. (A) Five × 106 EL4·IL-2 (ATCC, TIB 181) cells, cultured at a concentration of 0.5 × 106 cells per ml, were serum-starved for 16 h. Some of the starved cultures then were stimulated with IL-2 (100 units/ml) for 10 min as indicated. (Upper) In vitro kinase assays of Akt immunoprecipitated from Nonidet P-40 lysates of unstimulated or IL-2-stimulated EL4 cells, using the anti-Akt-CT antibody. (Lower) Anti-Akt-CT Western blot of the immunoprecipitates used in the kinase assay above. (B) Five × 106 BAF/3 cells stably transfected with the expression construct pdKCR-IL-2Rβ (3) cultured at a concentration of 0.5 × 106 cells per ml, were starved of IL-3 for 16 h. (Top and Middle) In vitro kinase assays carried out on anti-Akt immunoprecipitates of unstimulated and IL-2 (100 units/ml) or IL-3 (50 units/ml) stimulated cell lysates. (Bottom) Expression of c-akt as determined by probing Western blots of total cell lysates with the anti-Akt-CT antibody. (C) BAF/3 cells (5 × 106) stably expressing the IL-2Rβ or the IL-2Rβ·ΔS mutant, cultured at the concentration of 0.5 × 106 cells per ml, were transiently transfected with HA·Akt. Twenty-four hours after transfection, the cells were starved of IL-2 and IL-3 for 16 h. Subsequently, they were stimulated with IL-2 or IL-3 for 10 min as indicated. In vitro kinase assays were carried out on HA[A0]Akt immunoprecipitated from Nonidet P-40 cell lysates.
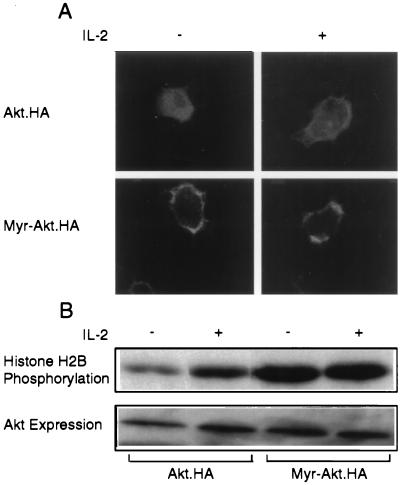
The PI3-kinase and the phosphoinositides it generates are localized at the cell membrane (25). We hypothesized therefore that membrane translocation could be a necessary step for the activation of Akt by growth factors. To address this hypothesis, a carboxyl-terminally tagged HA construct of wild-type Akt (Akt·HA) was transiently transfected into EL4 cells. After 16 h of serum starvation, starting at 24 h after the transfection, half of the transfected cultures were stimulated with IL-2. Ten minutes later, cytospins of both the unstimulated and IL-2-stimulated cultures were fixed with paraformaldehyde and stained with the anti-HA monoclonal antibody 12CA5. The results showed that Akt was localized in the cytosol, in the majority of the transiently transfected unstimulated cells. After IL-2 stimulation, however, the majority of Akt was detected on the cell membrane (Fig. 2A). Therefore, membrane translocation, perhaps due to the interaction of the Akt PH domain with PI3-kinase-generated phosphoinositides, may play an important role in the activation of Akt by IL-2. Strong support to this hypothesis was provided by the observation that an Akt hybrid molecule containing a c-src-derived myristylation signal (MGSSKSKPK) (26) at its N terminus is attached to the cell membrane (Fig. 2A) and is constitutively active (Fig. 2B).
Figure 2.
Akt activation by IL-2 is linked to the translocation of Akt to the cell membrane. (A) 107 EL4·IL-2 cells transiently transfected with carboxyl-terminally tagged Akt (Akt·HA) or myristylated Akt (Myr-Akt·HA) expression constructs and stained with the anti-HA monoclonal antibody 12CA5 and an anti-mouse fluorescein isthiocyanate-conjugated secondary antibody before and after treatment with IL-2. (Upper) Cells transfected with wild-type Akt. (Lower) Cells transfected with myristylated Akt. (B) In vitro kinase assays of Akt·HA and myristylated Akt·HA immunoprecipitated from Nonidet P-40 lysates of the cells described in A.
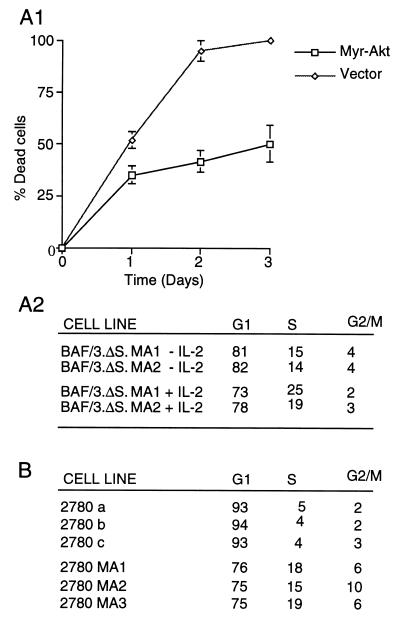
Like the parental BAF/3 cells, BAF/3-IL-2Rβ·ΔS cells undergo apoptosis within 48–72 h after IL-3 withdrawal, both in the presence and in the absence of IL-2 (10). Because IL-2 does not activate Akt in these cells (Fig. 1C), we hypothesized that its failure to rescue them from apoptosis induced by IL-3 withdrawal was due to its inability to activate Akt. To test this hypothesis, BAF/3 cells expressing IL-2Rβ·ΔS were transfected with CMV-6 or a CMV-6-based expression construct of myristylated Akt. Stably transfected cells were cultured in IL-3 deficient media in the presence or absence of IL-2. Live and dead cells then were counted daily for 3 days, and their numbers were used to calculate the ratio of dead/(dead + live) cells and the percentage of dead cells for each time point. Fig. 3A1 shows the mean values of the percentage of dead cells ± the SD at three daily time points for six independent mass cultures transfected with CMV-6 or myristylated Akt and maintained in the absence of IL-2. These data show that the myristylated Akt is sufficient to delay apoptosis induced by IL-3 withdrawal in BAF/3-IL-2Rβ·ΔS cells. The percentage of dead cells in cultures maintained in the presence of IL-2 was very similar to that of cultures maintained in the absence of IL-2 (data not shown), suggesting that the main IL-2 antiapoptotic signals are transduced via the S region of the IL-2Rβ and are mediated by Akt.
Figure 3.
Myristylated Akt inhibits apoptosis and cell cycle arrest induced by growth factor withdrawal in growth factor dependent lymphoid cells. (A1) Expression of myristylated Akt inhibits apoptosis induced by IL-2/IL-3 withdrawal in IL-2Rβ·ΔS expressing BAF/3 cells. Three independent cultures of BAF/3-IL-2Rβ·ΔS cells, stably transfected with a CMV-6 construct of myristylated Akt or the CMV-6 vector alone, were cultured in IL-2 and IL-3 deficient media at the concentration of 0.5 × 106 cells per ml. The percentage of dead cells over time was calculated from the number of live and dead cells counted daily as described in the text. (A2) Myristylated Akt inhibits G1 arrest of BAF/3-IL-2Rβ·ΔS cells surviving IL-3 withdrawal induced by IL-3 withdrawal. Two of the BAF/3 cell lines expressing IL-2Rβ·ΔS and myristylated Akt (BAF/3·ΔS·MA1 and MA2), described in A1, were exposed to ethidium bromide at 72 h (3 days) after growth factor withdrawal, and they were analyzed for DNA content by flow cytometry (5). The parental BAF/3 and BAF/3-IL-2Rβ·ΔS cells do not survive IL-3 withdrawal and die within 48–72 h even in the presence of IL-2. (B) 2780a T cells engineered to express myristylated Akt escape G1 arrest induced by IL-2 withdrawal. Three independent cultures of the IL-2-dependent rat T cell lymphoma line 2780a were infected with an SRα retrovirus vector-based construct of myristylated Akt, while three independent control cultures of the same cells were infected with the empty vector. Seventy-two hours after IL-2 withdrawal, the cells were analyzed for DNA content as in A2.
To determine whether the myristylated Akt-expressing cells that survive IL-3 withdrawal undergo cell cycle arrest or continue to cycle, cells were harvested at 72 h after IL-3 deprivation, and they were analyzed for DNA content by flow cytometry. The results (Fig. 3A2) showed that, at 72 h, surviving cells are distributed throughout the cell cycle. Cycling of these cells was confirmed by experiments demonstrating BrdUrd incorporation in the cell DNA (data not shown). Because BAF/3 cells engineered to express Bcl-2 also survive IL-3 withdrawal, but arrest in G1 (27), this finding suggests that Akt not only inhibits apoptosis but stimulates cell cycle progression. Exposure of BAF/3 cells expressing myristylated Akt and IL-2Rβ·ΔS to IL-2 further enhanced progression through the cell cycle (Fig. 3A2), suggesting that Akt complements the signaling defect of the IL-2Rβ·ΔS mutant and that IL-2 proliferative signals are transduced via both Akt-dependent and independent pathways. The effects of Akt on the cell cycle were confirmed in an IL-2 dependent T cell line (2780a), which undergoes G1 arrest rather than apoptosis after IL-2 withdrawal (20). Expression of myristylated Akt in these cells allowed them to escape G1 arrest induced by IL-2 deprivation (Fig. 3B).
The myristylated Akt mutant is constitutively localized at the cell membrane (Fig. 2A). To address the physiological nature of the biological effects of this mutant, we examined whether a PH domain Akt mutant (E40K) that exhibits enhanced basal kinase activity, but responds to physiological stimuli (A.B., N.N.A., T.O.C., and P.N.T., unpublished work), produces effects similar to those produced by myristylated Akt in BAF/3-IL-2Rβ·ΔS cells. The results (Table 1) showed that the Akt E40K mutant also inhibits apoptosis induced by IL-3 withdrawal. Its antiapoptotic activity, however, similarly to its basal kinase activity (data not shown), is lower than that of the myristylated Akt. In addition to its antiapoptotic effects, the Akt E40K mutant promoted cellular proliferation. Six mass cultures of BAF/3 cells independently transfected with Akt E40K, myristylated Akt, or the empty CMV-6 vector were cultured in IL-3-deficient media. The surviving cells in all the Akt E40K and myristylated Akt transfected cultures grew indefinitely in the absence of growth factors, giving rise to growth factor independent cell lines (data not shown).
Table 1.
Rate of apoptosis of BAF/3-IL-2Rβ·ΔS cells stably transfected with CMV-6 or CMV-6-based expression constructs of catalytically active Akt mutants
| Cells | Apoptosis, % dead cells
|
||
|---|---|---|---|
| 48 h | 72 h | 96 h | |
| BAF/3·ΔS CMV-6 | 74 ± 7.5 | 96 ± 2.9 | 100 |
| BAF/3·ΔS·E40K-Akt | 52 ± 3.5 | 66 ± 3.3 | 75 ± 3.2 |
| BAF/3·ΔS·Myr-Akt | 45 ± 4.1 | 52 ± 4.4 | 67 ± 4.5 |
Six independent cultures of BAF/3-IL-2Rβ·ΔS cells stably transfected with CMV-6 or CMV-6-based expression constructs of Akt E40K or myristylated Akt were cultured in the absence of IL-2 and IL-3 at the concentration of 0.5 × 106 cells per ml. Live and dead cells were counted daily and the percentage of dead cells (mean ± SD) at each time point was calculated as in Fig. 3A1. Surviving Akt E40K and myristylated Akt-expressing cells from all the cultures gave rise to growth factor-independent cell lines. Visible evidence of factor independent growth was observed within a week after growth factor deprivation.
The preceding data suggested that Akt is the mediator of IL-2 antiapoptotic and proliferative signals that are transduced via the S region of the IL-2Rβ. Because these IL-2 signals induce the expression of Bcl-2 and c-myc (10), we hypothesized that if Akt is a mediator of such signals it also would induce Bcl-2 and c-myc expression. To address this question Western blots of lysates of IL-2- and IL-3-starved BAF/3-IL-2Rβ·ΔS cells expressing myristylated Akt were probed with anti-Bcl-2 and anti-c-myc antibodies. Lysates of BAF/3-IL-2Rβ and BAF/3-IL-2Rβ·ΔS cells before and after IL-2 stimulation were used as controls. The results (Fig. 4A1 and B) confirmed that IL-2 induces expression of Bcl-2 and c-myc via signals transduced through the S region of the IL-2Rβ. Moreover, they showed that Akt induces the expression of both oncoproteins.
Figure 4.
Myristylated Akt replaces IL-2 signals transduced via the S region of the IL-2Rβ and induces Bcl-2 and c-myc expression in BAF/3-IL-2Rβ·ΔS cells. (A1) 106 BAF/3-IL-2Rβ or BAF/3-IL-2Rβ·ΔS cells were cultured at the concentration of 0.5 × 106 cells per ml in the absence of IL-2 or IL-3. Twenty-four hours later, the indicated cultures (lanes 2 and 4) were stimulated with IL-2 (100 units/ml) for 10 min. Unstimulated (lanes 1 and 3) and IL-2-stimulated (lanes 2 and 4) cells were lysed in the Nonidet P-40 lysis buffer. One × 106 cells from four independent BAF/3-IL-2Rβ·ΔS lines expressing a hemagglutinin-tagged myristylated Akt (MA1, MA2, MA3, and MA4) were cultured in parallel with the preceding cells also for 24 h in the absence of IL-2 and IL-3, and they were lysed in the same buffer. Western blots of all the lysates were probed with an anti-Bcl-2 rabbit polyclonal antiserum or with the anti-hemagglutinin tag antibody 12CA5. (A2) Five × 106 cells from four independent cultures of myristylated Akt-transfected, MA1, MA2, MA3, and MA4 and two of CMV-6-transfected BAF/3-IL-2Rβ·ΔS cells ΔS1 and ΔS2 were cultured in the presence of IL-2 (100 units per ml). At time 0, all cells were treated with staurosporine (2 μM). Cells were harvested at sequential time points as indicated, and live and dead cells were counted. The percentage of dead cells was calculated as described in Materials and Methods. (B) Western blots of the cell lysates, analyzed in A1, were probed with an anti-c-myc rabbit polyclonal antibody (Upstate Biotechnology) (dilution 1/2,000).
Earlier studies had shown that overexpression of Bcl-2 is sufficient to inhibit apoptosis in BAF/3 cells cultured in the absence of IL-3 (10, 27). The antiapoptotic role of Bcl-2 in Akt expressing BAF/3-IL-2Rβ.ΔS cells, suggested by these earlier studies, was further supported by experiments showing that these cells exhibit a delay in staurosporine-induced apoptosis (Fig. 4A2). Because apoptosis induced by staurosporine is delayed in cells overexpressing Bcl-2, but not in cells protected from apoptosis by other antiapoptotic signals (28), these data show that the induction of Bcl-2 in myristylated Akt-expressing cells is antiapoptotic. The Akt-mediated stimulation of the cell cycle, described in this report, appears to be mediated by the combined induction of Bcl-2 and c-myc as suggested by earlier studies, which had shown that overexpression of both Bcl-2 and c-myc in BAF/3 cells is sufficient to induce progression through the cell cycle (10).
DISCUSSION
The data in this report addressed several important questions regarding both the mechanism of activation of Akt by growth factors and the biological effects of the activated Akt. Our earlier studies and studies by others (12–16) had shown that Akt activation by growth factors depends on the PI3-kinase. More recent studies (17–19) provided support to the earlier suggestion that Akt is a direct target of the D3 phosphoinositides whose synthesis is catalyzed by the PI3-kinase (12). In this report we provide a link that was so far missing from the emerging scheme of Akt activation. Specifically, we show that, after growth factor stimulation, Akt translocates to the cell membrane. Therefore, the binding of the D3 phosphoinositides to the PH domain of Akt, suggested by the earlier studies, is likely to be responsible for membrane translocation. Moreover, because the myristylated Akt mutant, which is constitutively associated with the cell membrane is constitutively active, membrane translocation is likely to play an important role in Akt activation.
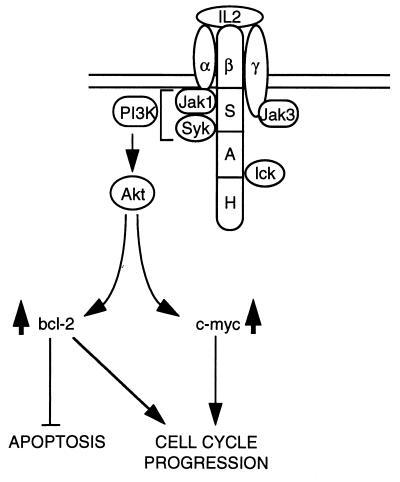
Hematopoietic growth factors promote cell viability and cellular proliferation (21, 29). Cells dependent on a given growth factor usually undergo apoptosis upon growth factor deprivation (16). In the case of IL-2, signals transduced by the S region of the IL-2Rβ via the PI3-kinase induce the expression of Bcl-2 and c-myc, and as a result they inhibit apoptosis and stimulate cellular proliferation (3, 30). In this report we showed that the inability of a deletion mutant of the IL-2Rβ (ΔS) to induce the expression of Bcl-2 and c-myc in response to IL-2, correlates with its inability to activate Akt. Moreover, we showed that catalytically active mutants of Akt induce expression of both Bcl-2 and c-myc. As a result, they inhibit apoptosis induced by growth factor withdrawal or staurosporine, stimulate progression through the cell cycle, and give rise to growth factor independent cell lines. Collectively, these data indicate that Akt transduces the IL-2 signals originating from the S region of the IL-2Rβ, which lead to Bcl-2 and c-myc expression, thus inhibiting apoptosis and stimulating cellular proliferation (Fig. 5).
Figure 5.
Model for the role of Akt in IL-2 signaling. Signals transduced from the S region of the IL-2Rβ activate the PI3-kinase and Akt and induce Bcl-2 and c-myc expression. These signals are sufficient to inhibit apoptosis and to stimulate progression through the cell cycle. Because catalytically active Akt mutants fully replace these IL-2 signals, we conclude that they are transduced via Akt.
Our earlier studies on Akt were centered primarily on the mechanism(s) of its activation (12, 17, 22). These studies identified Akt as a direct target of the PI3-kinase and suggested that it may contribute to the transduction of signals involved in a great variety of biological processes known to be PI3-kinase-dependent (31). The results presented here extend our earlier observations on Akt activation by showing that it is linked to the translocation of Akt to the cell membrane. In addition, they place Akt in a clearly defined pathway of IL-2 signaling. IL-2 signals transduced by Akt along this pathway regulate the expression of Bcl-2 and c-myc, and as a result they inhibit apoptosis and stimulate cellular proliferation.
Acknowledgments
We thank Dr. Zhao-Jun Liu and Prof. T. Taniguchi for the IL-2Rβ constructs and Dr. G.A. Evans for the BAF/3 cells. We also thank all the members of this laboratory for helpful discussions, Dr. Christine McMahon for the immunofluorescence studies, and Pat Bateman for secretarial assistance. This work was supported by U.S. Public Health A Service Grant CA57436. Additional support was provided by U.S. Public Health Service Grant CA06927 and an appropriation from the Commonwealth of Pennsylvania to the Fox Chase Cancer Center. N.N.A. was supported by a National Institutes of Health postdoctoral fellowship.
ABBREVIATIONS
- IL
interleukin
- IL-2R
IL-2 receptor
- PI3-kinase
phosphatidylinositol 3-kinase
- CMV
cytomegalovirus
References
- 1.Taniguchi T, Miyazaki T, Minami Y, Kawahara A, Fujii H, Nakagawa Y, Hatakeyama M, Liu Z J. Ann NY Acad Sci. 1995;766:235–244. doi: 10.1111/j.1749-6632.1995.tb26671.x. [DOI] [PubMed] [Google Scholar]
- 2.Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Silvennoinen O. Annu Rev Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 3.Giri J G, Ahdieh M, Eisenmann J, Shannebeck K, Grabstein K, Kumaki S, Namen A, Park L S, Cosman D, Anderson D. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sagamura K, Asao H, Kondo M, Tanaka N, Ishii N, Nakamura M, Takeshita T. Adv Immunol. 1995;59:225–277. doi: 10.1016/s0065-2776(08)60632-x. [DOI] [PubMed] [Google Scholar]
- 5.Minami Y, Kono T, Yamada K, Kobayashi N, Kawahara A, Perlmutter R M, Taniguchi T. EMBO J. 1993;12:759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami Y, Nakagawa Y, Kawahara A, Miyazaki T, Sada K, Yamamura H, Taniguchi T. Immunity. 1995;21:89–100. doi: 10.1016/1074-7613(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 7.Nelson B H, Lord J D, Greenberg P D. Mol Cell Biol. 1996;16:309–317. doi: 10.1128/mcb.16.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z J, Oishi I, Silvennoinen O, Witthuhn B A, Ihle J N, Taniguchi T. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 9.Ravichandran K S, Burakoff S J. J Biol Chem. 1994;269:1599–1602. [PubMed] [Google Scholar]
- 10.Miyazaki T, Junlin Z, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Perlmutter R M, Taniguchi T. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 11.Kanazawa T, Keeler M L, Varticovski L. Cell Immunol. 1994;156:378–388. doi: 10.1006/cimm.1994.1183. [DOI] [PubMed] [Google Scholar]
- 12.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N N, Franke F, Bellacosa A, Datta K, Gonzalez-Portal M E, Taguchi T, Testa J R, Tsichlis P N. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 14.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 15.Burgering B M, Coffer P J. Nature (London) 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 16.Downward J. Nature (London) 1995;376:553–554. doi: 10.1038/376553a0. [DOI] [PubMed] [Google Scholar]
- 17.Datta K, Bellacosa A, Chan T O, Tsichlis P N. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 18.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M A, Williams L T. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klippel A, Kavanaugh W M, Pot D, Williams L T. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazo P A, Klein-Szanto A J P, Tsichlis P N. J Virol. 1990;64:3948–3959. doi: 10.1128/jvi.64.8.3948-3959.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimes H L, Chan T O, Zweidler-McKay P A, Tong B, Tsichlis P N. Mol Cell Biol. 1996;16:6263–6272. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta K, Franke T F, Chan T O, Makris A, Yang S-I, Kaplan D, Morrison D K, Golemis E A, Tsichlis P N. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimes H L, Gilks C B, Chan T O, Porter S, Tsichlis P N. Proc Natl Acad Sci USA. 1996;93:14569–14573. doi: 10.1073/pnas.93.25.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhand R, Hara K, Hiles I, Bax B, Gout I, Panayotou G, Fry M J, Yonezawa K, Kasuga M, Waterfield M D. EMBO J. 1994;13:511–521. doi: 10.1002/j.1460-2075.1994.tb06289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens L T, Jackson T, Hawkins P. Biochim Biophys Acta. 1993;1179:27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 26.Pellman D, Garber E A, Cross F R, Hanafusa H. Nature (London) 1985;314:374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- 27.Marvel J, Perkins G P, Rivas A L, Collins M K L. Oncogene. 1994;9:1117–1122. [PubMed] [Google Scholar]
- 28.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 29.Loten J, Sachs L. Leukemia. 1996;10:925–931. [PubMed] [Google Scholar]
- 30.Satoh T, Minami Y, Kono T, Yamada K, Kawahara A, Taniguchi T, Kaziro Y. J Biol Chem. 1992;267:25423–25427. [PubMed] [Google Scholar]
- 31.Carpenter C L, Cantley L C. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]