Abstract
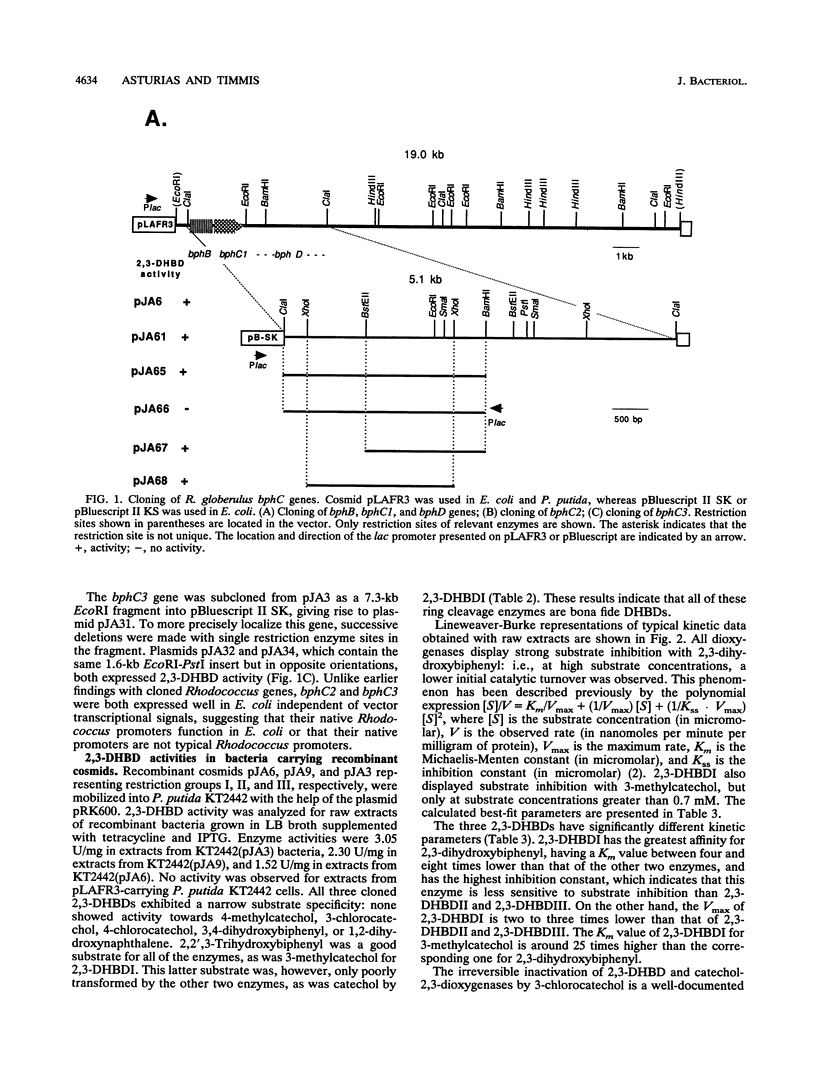
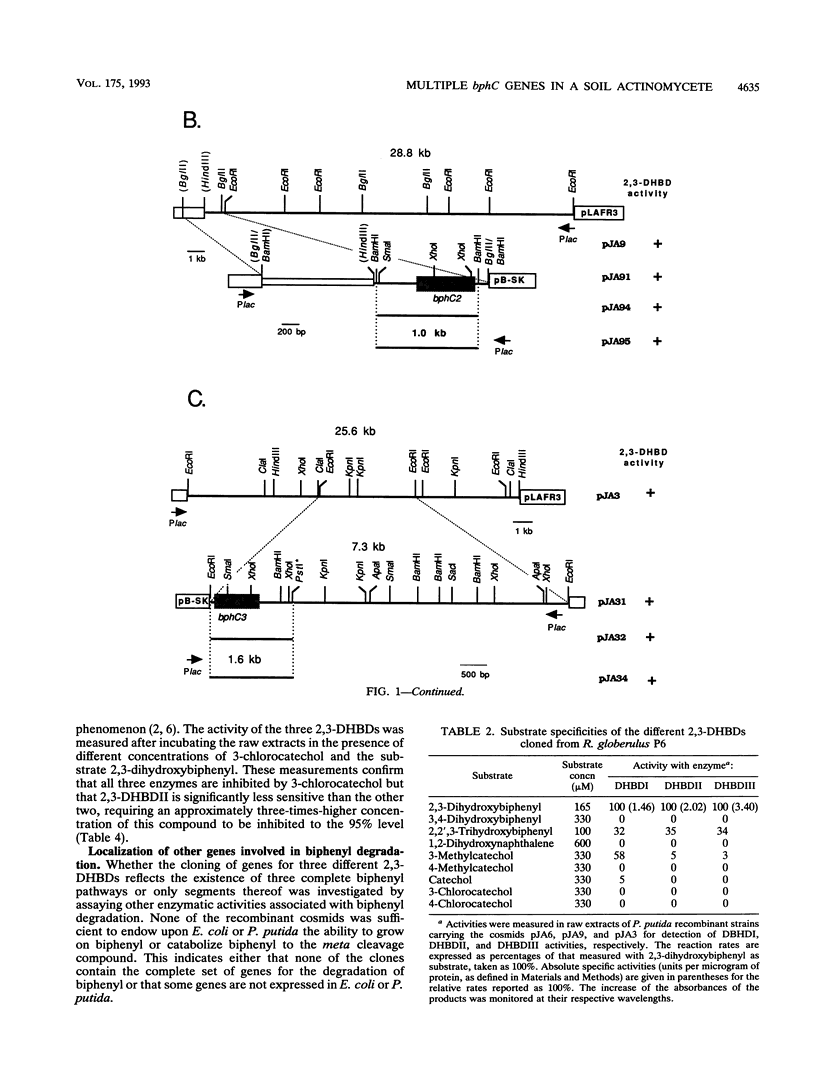
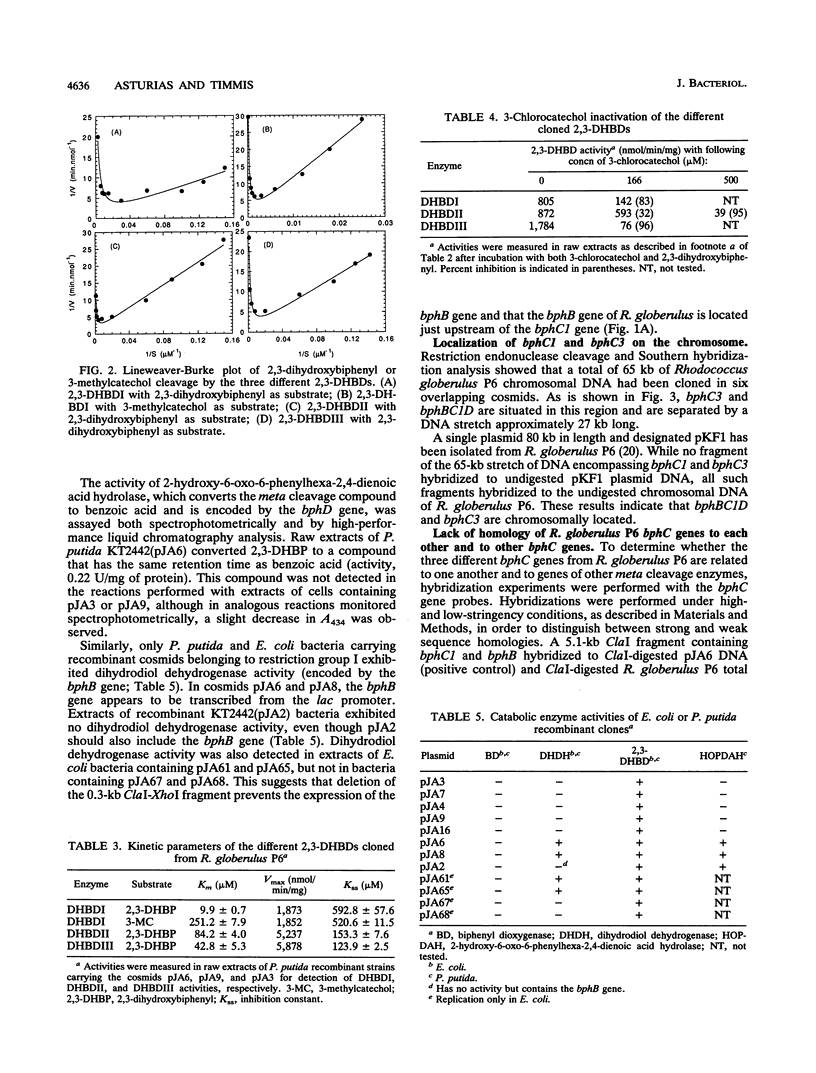
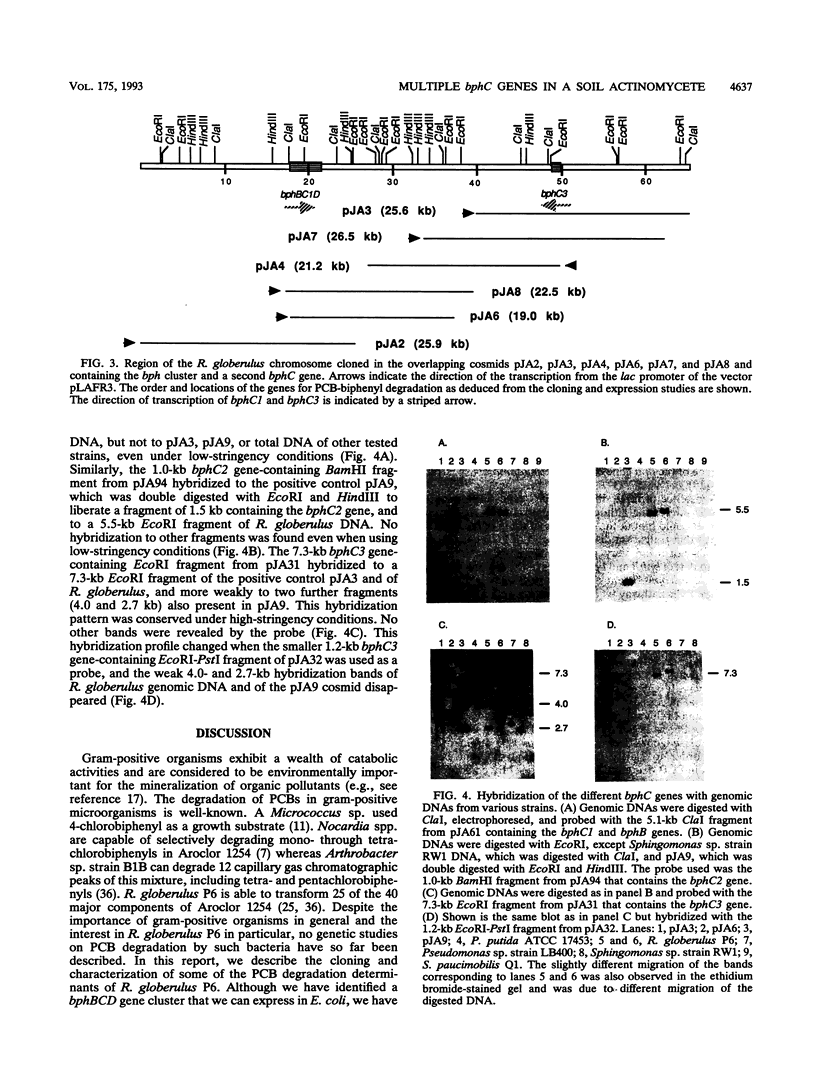
Rhodococcus globerulus P6 (previously designated Acinetobacter sp. strain P6, Arthrobacter sp. strain M5, and Corynebacterium sp. strain MB1) is able to degrade a wide range of polychlorinated biphenyl (PCB) congeners. The genetic and biochemical analyses of the PCB catabolic pathway reported here have revealed the existence of a PCB gene cluster--bphBC1D--and two further bphC genes--bphC2 and bphC3--that encode three narrow-substrate-specificity enzymes (2,3-dihydroxybiphenyl dioxygenases) that meta cleave the first aromatic ring. None of the bphC genes show by hybridization homology to each other or to bphC genes in other bacteria, and the three bphC gene products have different kinetic parameters and sensitivities to inactivation by 3-chlorocatechol. This suggests that there exists a wide diversity in PCB meta cleavage enzymes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. H., Huang C. M., Higson F. K., Brenner V., Focht D. D. Construction of a 3-chlorobiphenyl-utilizing recombinant from an intergeneric mating. Appl Environ Microbiol. 1992 Feb;58(2):647–654. doi: 10.1128/aem.58.2.647-654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad D., Massé R., Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni: homology to polychlorobiphenyl-degrading genes in other bacteria. Gene. 1990 Jan 31;86(1):53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R. A., Gilbert P. E., Lidgett R. A., Mainprize J. H., Vodden H. A. The degradation of polychlorinated biphenyls by micro-organisms. Sci Total Environ. 1975 May;4(1):53–61. doi: 10.1016/0048-9697(75)90014-5. [DOI] [PubMed] [Google Scholar]
- Bedard D. L., Unterman R., Bopp L. H., Brennan M. J., Haberl M. L., Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986 Apr;51(4):761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Wagner R. E., Brennan M. J., Haberl M. L., Brown J. F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullum J., Altenbuchner J., Flett F., Piendl W. DNA amplification and genetic instability in Streptomyces. Biotechnol Genet Eng Rev. 1986;4:59–78. doi: 10.1080/02648725.1986.10647823. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltis L. D., Hofmann B., Hecht H. J., Lünsdorf H., Timmis K. N. Purification and crystallization of 2,3-dihydroxybiphenyl 1,2-dioxygenase. J Biol Chem. 1993 Feb 5;268(4):2727–2732. [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty W. R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Bagdasarian M., Bagdasarian M. M., Timmis K. N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Arimura N. Purification and properties of 2,3-dihydroxybiphenyl dioxygenase from polychlorinated biphenyl-degrading Pseudomonas pseudoalcaligenes and Pseudomonas aeruginosa carrying the cloned bphC gene. J Bacteriol. 1987 Feb;169(2):924–927. doi: 10.1128/jb.169.2.924-927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Chakrabarty A. M. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982 Sep;44(3):619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Hayase N., Taira K., Tomizuka N. Molecular relationship of chromosomal genes encoding biphenyl/polychlorinated biphenyl catabolism: some soil bacteria possess a highly conserved bph operon. J Bacteriol. 1989 Oct;171(10):5467–5472. doi: 10.1128/jb.171.10.5467-5472.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986 May;166(2):392–398. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Simon J. R., Chakrabarty A. M. Common induction and regulation of biphenyl, xylene/toluene, and salicylate catabolism in Pseudomonas paucimobilis. J Bacteriol. 1983 Jun;154(3):1356–1362. doi: 10.1128/jb.154.3.1356-1362.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Metabolic breakdown of Kaneclors (polychlorobiphenyls) and their products by Acinetobacter sp. Appl Environ Microbiol. 1983 Jul;46(1):140–145. doi: 10.1128/aem.46.1.140-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Herrero M., de Lorenzo V., Timmis K. N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990 Nov;172(11):6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabisch M., Fortnagel P. Nucleotide sequence of metapyrocatechase I (catechol 2,3-oxygenase I) gene mpcI from Alcaligenes eutrophus JMP222. Nucleic Acids Res. 1990 Jun 11;18(11):3405–3406. doi: 10.1093/nar/18.11.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabisch M., Fortnagel P. Nucleotide sequence of the metapyrocatechase II (catechol 2,3-oxygenase II) gene mpcII from Alcaligenes eutrophus JMP 222. Nucleic Acids Res. 1990 Sep 25;18(18):5543–5543. doi: 10.1093/nar/18.18.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil H., Lebens M. R., Williams P. A. TOL plasmid pWW15 contains two nonhomologous, independently regulated catechol 2,3-oxygenase genes. J Bacteriol. 1985 Jul;163(1):248–255. doi: 10.1128/jb.163.1.248-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler B., de Lorenzo V., Timmis K. N. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992 May;233(1-2):293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- Khan A., Tewari R., Walia S. Molecular cloning of 3-phenylcatechol dioxygenase involved in the catabolic pathway of chlorinated biphenyl from Pseudomonas putida and its expression in Escherichia coli. Appl Environ Microbiol. 1988 Nov;54(11):2664–2671. doi: 10.1128/aem.54.11.2664-2671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Walia S. Cloning of bacterial genes specifying degradation of 4-chlorobiphenyl from Pseudomonas putida OU83. Appl Environ Microbiol. 1989 Apr;55(4):798–805. doi: 10.1128/aem.55.4.798-805.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Cometabolism of polychlorinated biphenyls: enhanced transformation of Aroclor 1254 by growing bacterial cells. Appl Environ Microbiol. 1988 Aug;54(8):1940–1945. doi: 10.1128/aem.54.8.1940-1945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhm A. E., Stolz A., Ngai K. L., Knackmuss H. J. Purification and characterization of a 1,2-dihydroxynaphthalene dioxygenase from a bacterium that degrades naphthalenesulfonic acids. J Bacteriol. 1991 Jun;173(12):3795–3802. doi: 10.1128/jb.173.12.3795-3802.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna M., Someda K., Morita K., Yamanouchi Y., Kurimoto T., Kawamura Y., Matsumura H. [Ischemic cerebral symptoms after subarachnoid hemorrhage due to aneurysmal rupture (author's transl)]. No Shinkei Geka. 1978 Jun;6(6):543–548. [PubMed] [Google Scholar]
- Mondello F. J. Cloning and expression in Escherichia coli of Pseudomonas strain LB400 genes encoding polychlorinated biphenyl degradation. J Bacteriol. 1989 Mar;171(3):1725–1732. doi: 10.1128/jb.171.3.1725-1732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Schreiner A., Fuchs K., Lottspeich F., Poth H., Lingens F. Degradation of 2-methylaniline in Rhodococcus rhodochrous: cloning and expression of two clustered catechol 2,3-dioxygenase genes from strain CTM. J Gen Microbiol. 1991 Aug;137(8):2041–2048. doi: 10.1099/00221287-137-8-2041. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taira K., Hayase N., Arimura N., Yamashita S., Miyazaki T., Furukawa K. Cloning and nucleotide sequence of the 2,3-dihydroxybiphenyl dioxygenase gene from the PCB-degrading strain of Pseudomonas paucimobilis Q1. Biochemistry. 1988 May 31;27(11):3990–3996. doi: 10.1021/bi00411a015. [DOI] [PubMed] [Google Scholar]
- Unterman R., Bedard D. L., Brennan M. J., Bopp L. H., Mondello F. J., Brooks R. E., Mobley D. P., McDermott J. B., Schwartz C. C., Dietrich D. K. Biological approaches for polychlorinated biphenyl degradation. Basic Life Sci. 1988;45:253–269. doi: 10.1007/978-1-4899-0824-7_17. [DOI] [PubMed] [Google Scholar]
- Wittich R. M., Wilkes H., Sinnwell V., Francke W., Fortnagel P. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl Environ Microbiol. 1992 Mar;58(3):1005–1010. doi: 10.1128/aem.58.3.1005-1010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R., Mondello F. J. Sequence similarities in the genes encoding polychlorinated biphenyl degradation by Pseudomonas strain LB400 and Alcaligenes eutrophus H850. J Bacteriol. 1989 Mar;171(3):1733–1735. doi: 10.1128/jb.171.3.1733-1735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



