Abstract
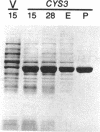
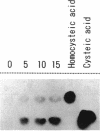
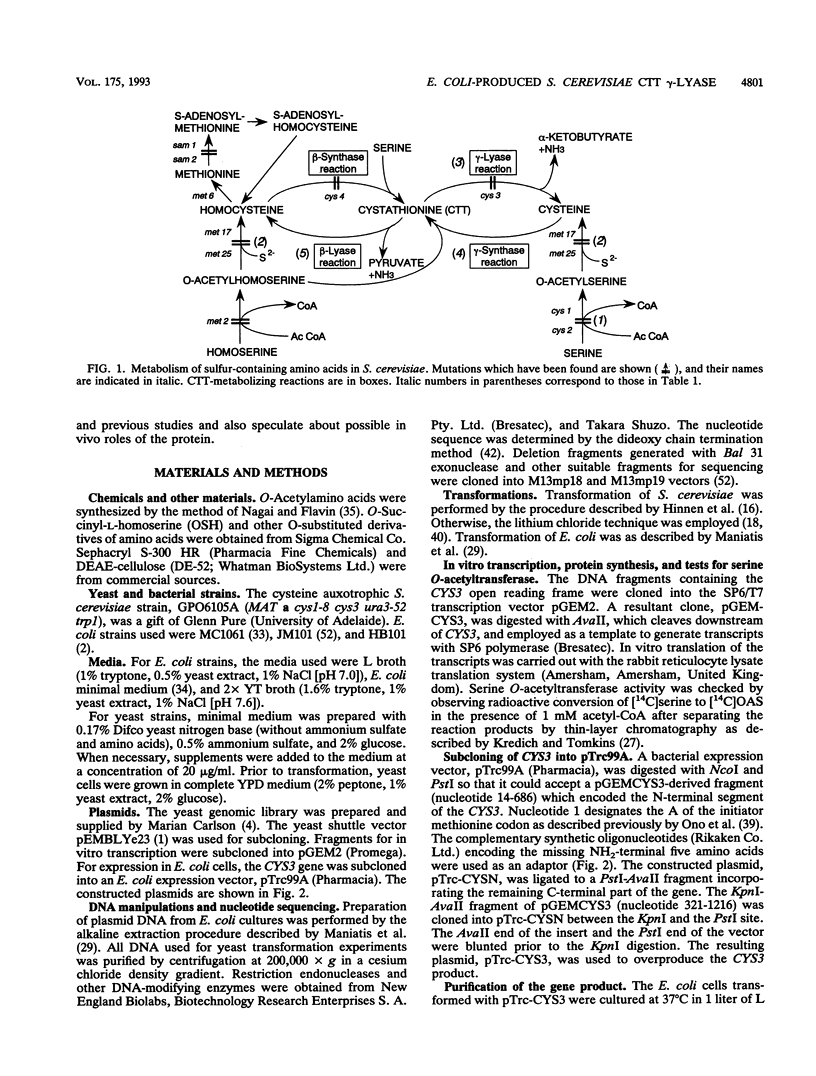
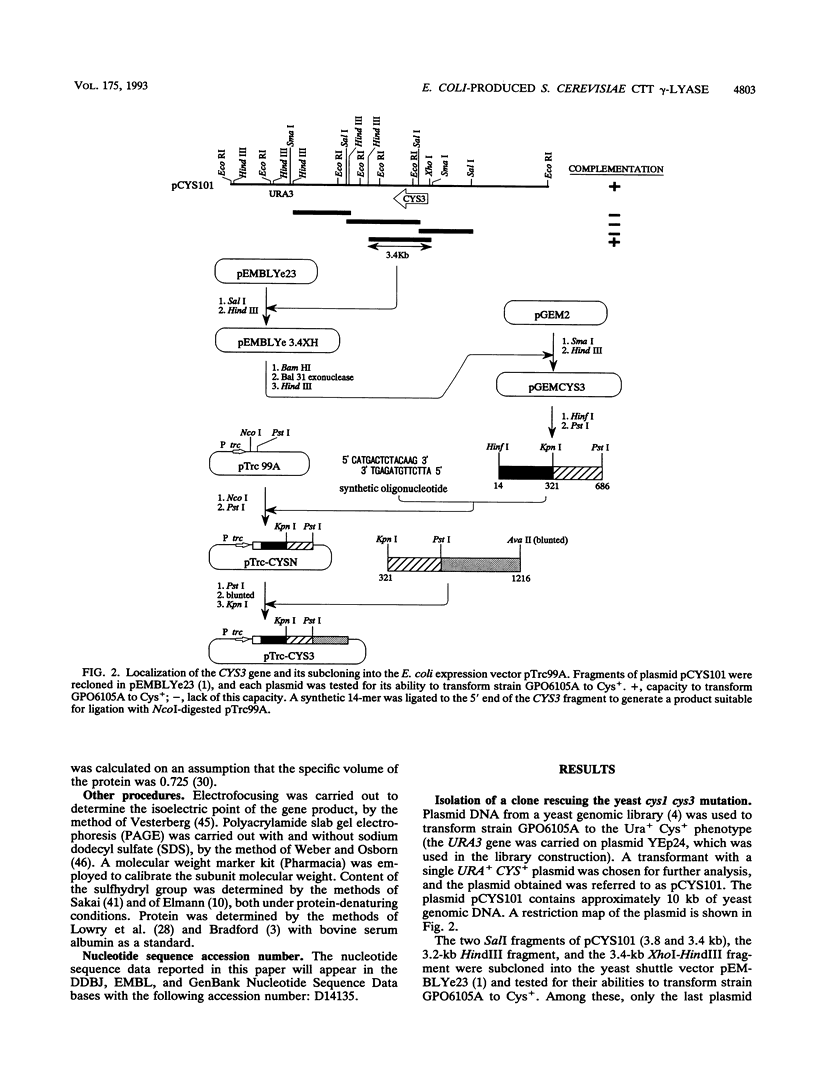
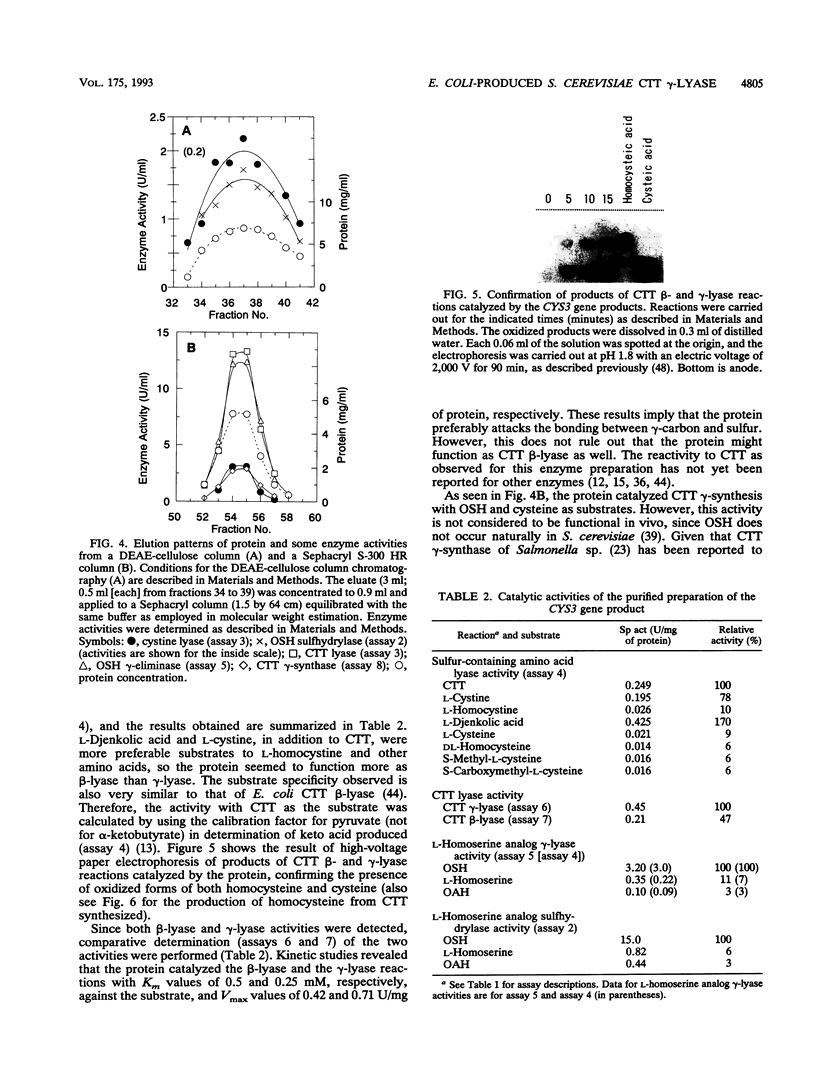
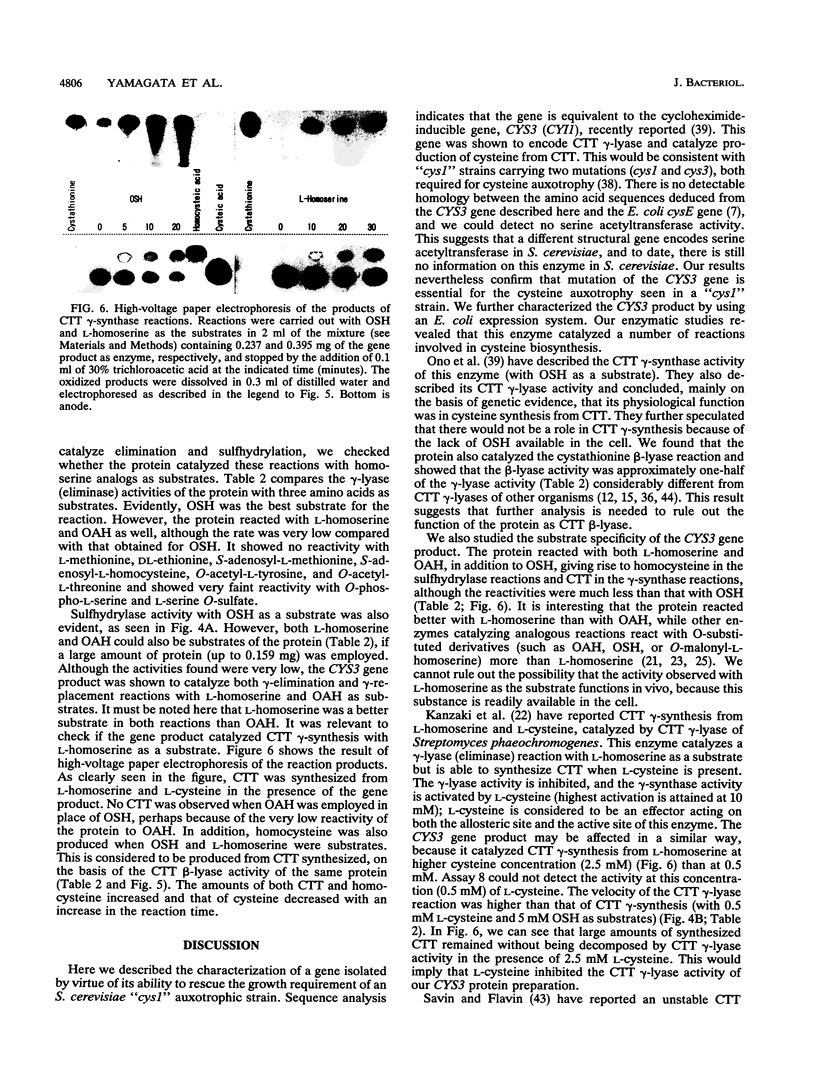
By screening a yeast genomic library, we isolated and characterized a gene rescuing the cysteine requirement in a "cys1" strain of Saccharomyces cerevisiae. Except for four residues in the open reading frame composed of 1,182 nucleotides, the DNA sequence was the same as that for the CYS3 (CYI1) gene, encoding cystathionine gamma-lyase (EC 4.4.1.1), and isolated previously as a cycloheximide-induced gene (B. Ono, K. Tanaka, K. Naito, C. Heike, S. Shinoda, S. Yamamoto, S. Ohmori, T. Oshima, and A. Toh-e, J. Bacteriol. 174:pp.3339-3347, 1992). S. cerevisiae "cys1" strains carry two closely linked mutations; one (cys1) causes a defect in serine O-acetyltransferase (EC 2.3.1.30), and another, designated cys3, impairs cystathionine gamma-lyase activity. Rescue of the cysteine requirement by the gene encoding cystathionine gamma-lyase is consistent with both defects being responsible for the cysteine auxotrophy. In an effort to further determine the physicochemical and enzymatic properties of this enzyme, a coding fragment was cloned into an Escherichia coli expression plasmid, and the protein was produced in the bacteria. The induced protein was extracted by sonication and purified to homogeneity through one course of DEAE-cellulose column chromatography. The yield of the protein was approximately 150 mg from cells cultured in 1 liter of L broth. The protein showed molecular weights of approximately 194,000 and 48,000 (for the subunit), suggesting a tetrameric structure. An s20,w value of 8.8 was estimated by centrifugation in a sucrose concentration gradient. No sulfhydryl groups were detected, which is consistent with the absence of cysteine residues in the coding sequence. The isoelectric point was at pH 5.2. The protein showed a number of cystathionine-related activities, i.e., cystathionine beta-lyase (EC 4.4.1.8), cystathionine gamma-lyase, and cystathionine gamma-synthase (EC 4.2.99.9) with L-homoserine as substrate. In addition, we demonstrated L-homoserine sulfhydrylase (adding H2S) activity but could find no detectable serine O-acteyltransferease activity. In this paper, we compare the enzymatic properties of the protein with those of homologous enzymes previously reported and discuss the possibility that this enzyme has a physiological role as cystathionine Beta-lyase and cystathionine gamma-synthase in addition to its previously described role as cystathionine gamma-lyase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldari C., Cesareni G. Plasmids pEMBLY: new single-stranded shuttle vectors for the recovery and analysis of yeast DNA sequences. Gene. 1985;35(1-2):27–32. doi: 10.1016/0378-1119(85)90154-4. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Geever R. F., Asch D. K., Case M. E. Sequence and characterization of the met-7 gene of Neurospora crassa. Gene. 1992 Feb 15;111(2):265–266. doi: 10.1016/0378-1119(92)90698-o. [DOI] [PubMed] [Google Scholar]
- D'Andrea R., Surdin-Kerjan Y., Pure G., Cherest H. Molecular genetics of met 17 and met 25 mutants of Saccharomyces cerevisiae: intragenic complementation between mutations of a single structural gene. Mol Gen Genet. 1987 Apr;207(1):165–170. doi: 10.1007/BF00331505. [DOI] [PubMed] [Google Scholar]
- Denk D., Böck A. L-cysteine biosynthesis in Escherichia coli: nucleotide sequence and expression of the serine acetyltransferase (cysE) gene from the wild-type and a cysteine-excreting mutant. J Gen Microbiol. 1987 Mar;133(3):515–525. doi: 10.1099/00221287-133-3-515. [DOI] [PubMed] [Google Scholar]
- Duchange N., Zakin M. M., Ferrara P., Saint-Girons I., Park I., Tran S. V., Py M. C., Cohen G. N. Structure of the metJBLF cluster in Escherichia coli K12. Sequence of the metB structural gene and of the 5'- and 3'-flanking regions of the metBL operon. J Biol Chem. 1983 Dec 25;258(24):14868–14871. [PubMed] [Google Scholar]
- Dwivedi C. M., Ragin R. C., Uren J. R. Cloning, purification, and characterization of beta-cystathionase from Escherichia coli. Biochemistry. 1982 Jun 22;21(13):3064–3069. doi: 10.1021/bi00256a005. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erickson P. F., Maxwell I. H., Su L. J., Baumann M., Glode L. M. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990 Jul 15;269(2):335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook E. L., Greene R. C., Krueger J. H. Purification and properties of cystathionine gamma-synthase from overproducing strains of Escherichia coli. Biochemistry. 1990 Jan 16;29(2):435–442. doi: 10.1021/bi00454a019. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki H., Kobayashi M., Nagasawa T., Yamada H. Purification and characterization of cystathionine gamma-synthase type II from Bacillus sphaericus. Eur J Biochem. 1987 Feb 16;163(1):105–112. doi: 10.1111/j.1432-1033.1987.tb10742.x. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Cherest H., Surdin-Kerjan Y. Nucleotide sequence of the Saccharomyces cerevisiae MET25 gene. Nucleic Acids Res. 1986 Oct 24;14(20):7861–7871. doi: 10.1093/nar/14.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. The regulation of methionine synthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970 Apr 10;245(7):1842–1855. [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Masselot M., De Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Mol Gen Genet. 1975 Aug 5;139(2):121–132. doi: 10.1007/BF00264692. [DOI] [PubMed] [Google Scholar]
- Masselot M., Surdin-Kerjan Y. Methionine biosynthesis in Saccharomyces cerevisiae. II. Gene-enzyme relationships in the sulfate assimilation pathway. Mol Gen Genet. 1977 Jul 7;154(1):23–30. doi: 10.1007/BF00265572. [DOI] [PubMed] [Google Scholar]
- Meissner P. S., Sisk W. P., Berman M. L. Bacteriophage lambda cloning system for the construction of directional cDNA libraries. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4171–4175. doi: 10.1073/pnas.84.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Kanzaki H., Yamada H. Cystathionine gamma-lyase from Streptomyces phaeochromogenes. Methods Enzymol. 1987;143:486–492. doi: 10.1016/0076-6879(87)43087-5. [DOI] [PubMed] [Google Scholar]
- Ono B., Shirahige Y., Nanjoh A., Andou N., Ohue H., Ishino-Arao Y. Cysteine biosynthesis in Saccharomyces cerevisiae: mutation that confers cystathionine beta-synthase deficiency. J Bacteriol. 1988 Dec;170(12):5883–5889. doi: 10.1128/jb.170.12.5883-5889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B., Suruga T., Yamamoto M., Yamamoto S., Murata K., Kimura A., Shinoda S., Ohmori S. Cystathionine accumulation in Saccharomyces cerevisiae. J Bacteriol. 1984 Jun;158(3):860–865. doi: 10.1128/jb.158.3.860-865.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B., Tanaka K., Naito K., Heike C., Shinoda S., Yamamoto S., Ohmori S., Oshima T., Toh-e A. Cloning and characterization of the CYS3 (CYI1) gene of Saccharomyces cerevisiae. J Bacteriol. 1992 May;174(10):3339–3347. doi: 10.1128/jb.174.10.3339-3347.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H. Quantitative microdetermination of total--SH groups in proteins. Anal Biochem. 1968 Nov;26(2):269–276. doi: 10.1016/0003-2697(68)90337-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin M. A., Flavin M. Cystationine synthesis in yeast: an alternative pathway for homocysteine biosynthesis. J Bacteriol. 1972 Oct;112(1):299–303. doi: 10.1128/jb.112.1.299-303.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren J. R. Cystathionine beta-lyase from Escherichia coli. Methods Enzymol. 1987;143:483–486. doi: 10.1016/0076-6879(87)43086-3. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamagata S. O-Acetylserine and O-acetylhomoserine sulfhydrylase of yeast. Subunit structure. J Biochem. 1976 Oct;80(4):787–797. doi: 10.1093/oxfordjournals.jbchem.a131339. [DOI] [PubMed] [Google Scholar]
- Yamagata S. Partial purification and some properties of homoserine O-acetyltransferase of a methionine auxotroph of Saccharomyces cerevisiae. J Bacteriol. 1987 Aug;169(8):3458–3463. doi: 10.1128/jb.169.8.3458-3463.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. Evidence for the identity of O-acetylserine sulfhydrylase with O-acetylhomoserine sulfhydrylase in yeast. J Biochem. 1974 Jun;75(6):1221–1229. doi: 10.1093/oxfordjournals.jbchem.a130505. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. O-acetylserine and O-acetylhomoserine sulfhydrylase of yeast; studies with methionine auxotrophs. J Biochem. 1975 May;77(5):1029–1036. doi: 10.1093/oxfordjournals.jbchem.a130803. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]