Abstract
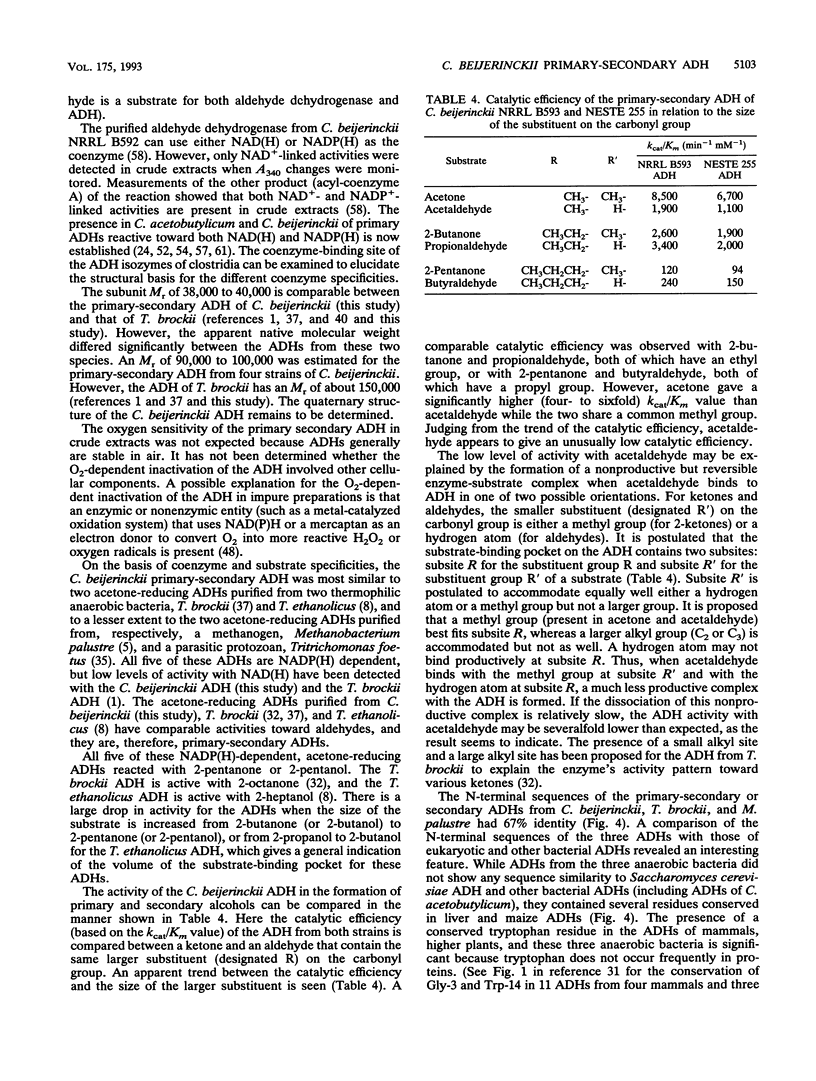
Two primary alcohols (1-butanol and ethanol) are major fermentation products of several clostridial species. In addition to these two alcohols, the secondary alcohol 2-propanol is produced to a concentration of about 100 mM by some strains of Clostridium beijerinckii. An alcohol dehydrogenase (ADH) has been purified to homogeneity from two strains (NRRL B593 and NESTE 255) of 2-propanol-producing C. beijerinckii. When exposed to air, the purified ADH was stable, whereas the partially purified ADH was inactivated. The ADHs from the two strains had similar structural and kinetic properties. Each had a native M(r) of between 90,000 and 100,000 and a subunit M(r) of between 38,000 and 40,000. The ADHs were NADP(H) dependent, but a low level of NAD(+)-linked activity was detected. They were equally active in reducing aldehydes and 2-ketones, but a much lower oxidizing activity was obtained with primary alcohols than with secondary alcohols. The kcat/Km value for the alcohol-forming reaction appears to be a function of the size of the larger alkyl substituent on the carbonyl group. ADH activities measured in the presence of both acetone and butyraldehyde did not exceed activities measured with either substrate present alone, indicating a common active site for both substrates. There was no similarity in the N-terminal amino acid sequence between that of the ADH and those of fungi and several other bacteria. However, the N-terminal sequence had 67% identity with those of two other anaerobes, Thermoanaerobium brockii and Methanobacterium palustre. Furthermore, conserved glycine and tryptophan residues are present in ADHs of these three anaerobic bacteria and ADHs of mammals and green plants.
Full text
PDF


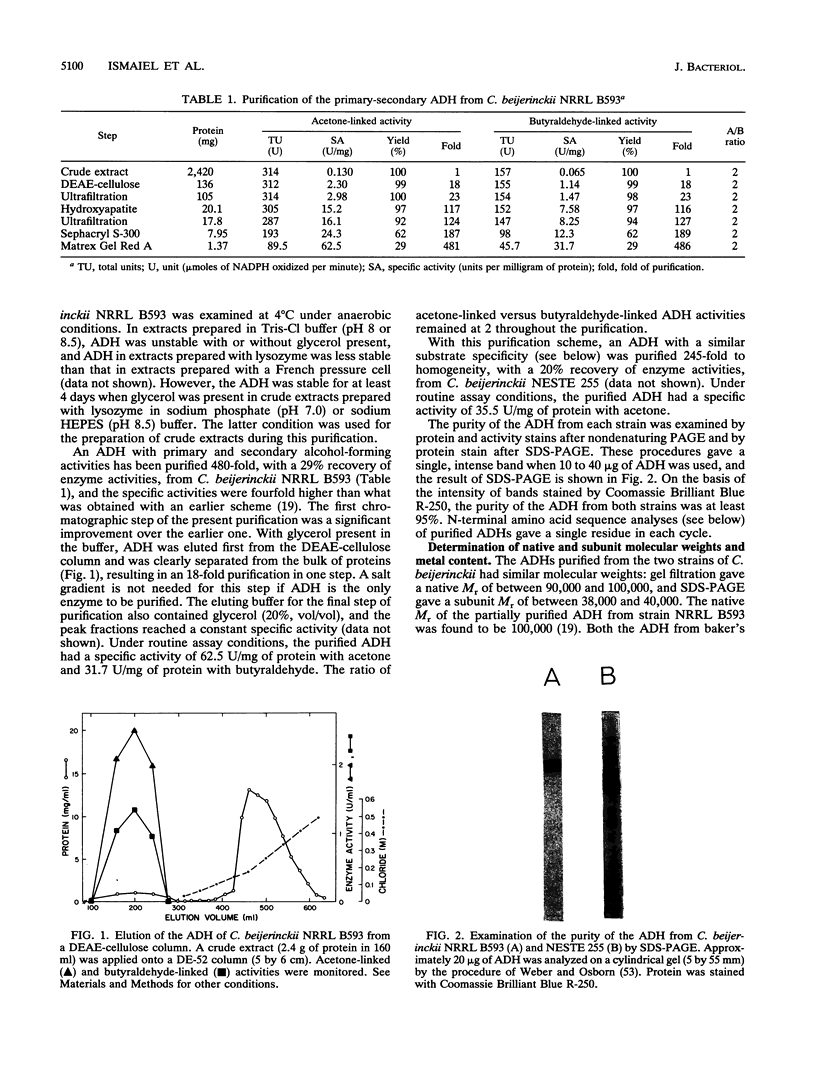
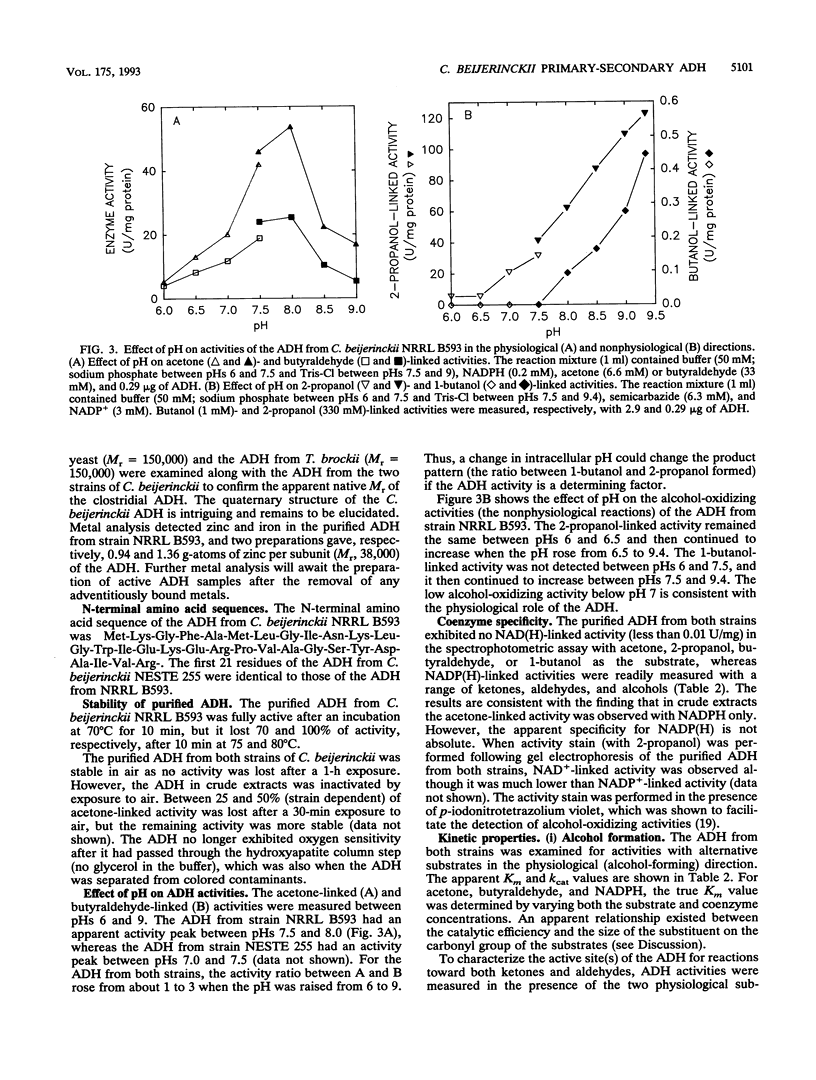
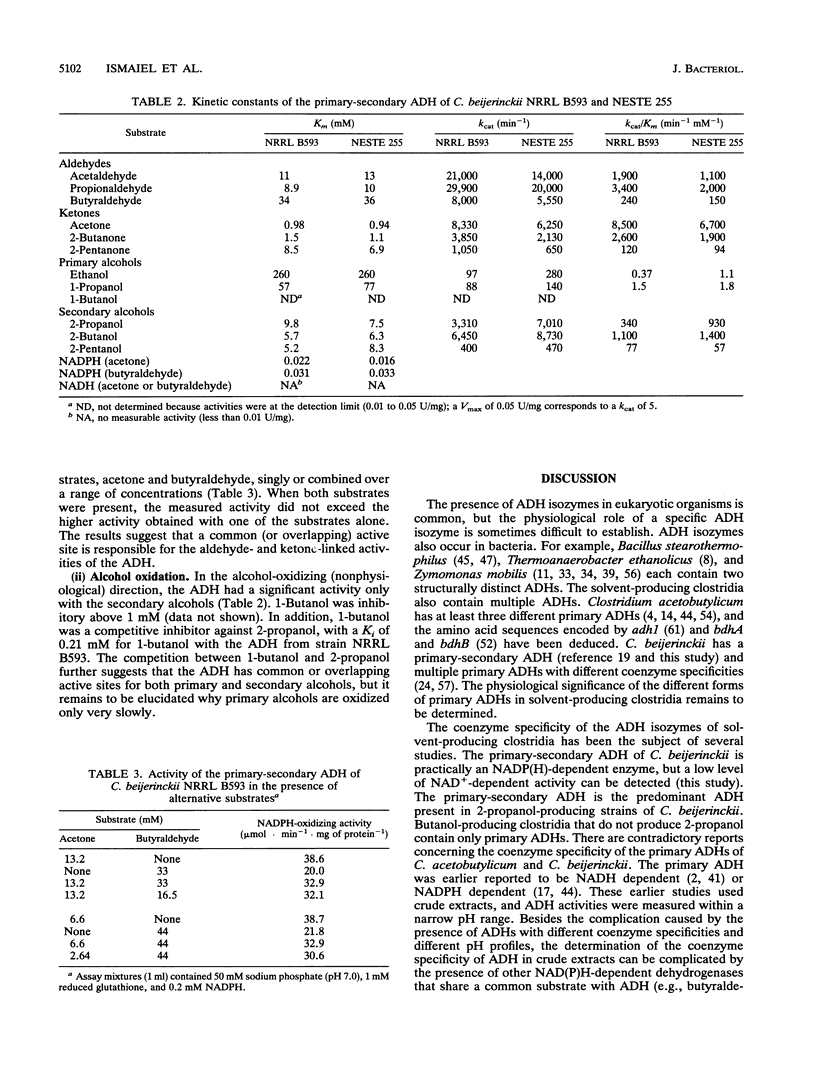


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Bleicher K., Winter J. Purification and properties of F420- and NADP(+)-dependent alcohol dehydrogenases of Methanogenium liminatans and Methanobacterium palustre, specific for secondary alcohols. Eur J Biochem. 1991 Aug 15;200(1):43–51. doi: 10.1111/j.1432-1033.1991.tb21046.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryant F. O., Wiegel J., Ljungdahl L. G. Purification and Properties of Primary and Secondary Alcohol Dehydrogenases from Thermoanaerobacter ethanolicus. Appl Environ Microbiol. 1988 Feb;54(2):460–465. doi: 10.1128/aem.54.2.460-465.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. P., Perry J. J. Purification and characterization of the secondary alcohol dehydrogenase from propane-utilizing Mycobacterium vaccae strain JOB-5. J Gen Microbiol. 1985 Nov;131(11):2901–2907. doi: 10.1099/00221287-131-11-2901. [DOI] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Osman Y. A., Ingram L. O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987 Jun;169(6):2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem J. 1966 Jul;100(1):34–46. doi: 10.1042/bj1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund H., Müller-Wille P., Horjales E., Futer O., Holmquist B., Vallee B. L., Hög J. O., Kaiser R., Jörnvall H. Comparison of three classes of human liver alcohol dehydrogenase. Emphasis on different substrate binding pockets. Eur J Biochem. 1990 Oct 24;193(2):303–310. doi: 10.1111/j.1432-1033.1990.tb19337.x. [DOI] [PubMed] [Google Scholar]
- George H. A., Chen J. S. Acidic Conditions Are Not Obligatory for Onset of Butanol Formation by Clostridium beijerinckii (Synonym, C. butylicum). Appl Environ Microbiol. 1983 Aug;46(2):321–327. doi: 10.1128/aem.46.2.321-327.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Johnson J. L., Moore W. E., Holdeman L. V., Chen J. S. Acetone, Isopropanol, and Butanol Production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microbiol. 1983 Mar;45(3):1160–1163. doi: 10.1128/aem.45.3.1160-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiu S. F., Zhu C. X., Yan R. T., Chen J. S. Butanol-Ethanol Dehydrogenase and Butanol-Ethanol-Isopropanol Dehydrogenase: Different Alcohol Dehydrogenases in Two Strains of Clostridium beijerinckii (Clostridium butylicum). Appl Environ Microbiol. 1987 Apr;53(4):697–703. doi: 10.1128/aem.53.4.697-703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barist I., Barnabe N. Thermostable NAD-linked secondary alcohol dehydrogenase from propane-grown Pseudomonas fluorescens NRRL B-1244. Appl Environ Microbiol. 1983 Jul;46(1):98–105. doi: 10.1128/aem.46.1.98-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ikuta T., Szeto S., Yoshida A. Three human alcohol dehydrogenase subunits: cDNA structure and molecular and evolutionary divergence. Proc Natl Acad Sci U S A. 1986 Feb;83(3):634–638. doi: 10.1073/pnas.83.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrossek D., Steinbüchel A., Schlegel H. G. Alcohol dehydrogenase gene from Alcaligenes eutrophus: subcloning, heterologous expression in Escherichia coli, sequencing, and location of Tn5 insertions. J Bacteriol. 1988 Nov;170(11):5248–5256. doi: 10.1128/jb.170.11.5248-5256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. The primary structure of the protein chain of the ethanol-active isoenzyme. Eur J Biochem. 1970 Sep;16(1):25–40. doi: 10.1111/j.1432-1033.1970.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Hög J. O., Von Bahr-Lindström H., Johansson J., Kaiser R., Persson B. Alcohol dehydrogenases and aldehyde dehydrogenases. Biochem Soc Trans. 1988 Jun;16(3):223–227. doi: 10.1042/bst0160223. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson B., Jeffery J. Characteristics of alcohol/polyol dehydrogenases. The zinc-containing long-chain alcohol dehydrogenases. Eur J Biochem. 1987 Sep 1;167(2):195–201. doi: 10.1111/j.1432-1033.1987.tb13323.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Keshav K. F., Yomano L. P., An H. J., Ingram L. O. Cloning of the Zymomonas mobilis structural gene encoding alcohol dehydrogenase I (adhA): sequence comparison and expression in Escherichia coli. J Bacteriol. 1990 May;172(5):2491–2497. doi: 10.1128/jb.172.5.2491-2497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. E., Johnston M. Purification and properties of a secondary alcohol dehydrogenase from the parasitic protozoan Tritrichomonas foetus. J Biol Chem. 1985 Jul 5;260(13):8038–8043. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale A. D., Scopes R. K., Kelly J. M., Wettenhall R. E. The two alcohol dehydrogenases of Zymomonas mobilis. Purification by differential dye ligand chromatography, molecular characterisation and physiological roles. Eur J Biochem. 1986 Jan 2;154(1):119–124. doi: 10.1111/j.1432-1033.1986.tb09366.x. [DOI] [PubMed] [Google Scholar]
- Peretz M., Burstein Y. Amino acid sequence of alcohol dehydrogenase from the thermophilic bacterium Thermoanaerobium brockii. Biochemistry. 1989 Aug 8;28(16):6549–6555. doi: 10.1021/bi00442a004. [DOI] [PubMed] [Google Scholar]
- Petitdemange H., Desbordes J., Berthelin J., Gay R. Conversion enzymatique du n-butyraldéhyde en n-butanol chez Clostridium acétobutylicum. C R Acad Sci Hebd Seances Acad Sci D. 1968 Apr 22;266(17):1772–1774. [PubMed] [Google Scholar]
- Runswick M. J., Harris J. I. Purification of alcohol dehydrogenase from Bacillus stearothermophilus by affinity chromatography. FEBS Lett. 1978 Aug 15;92(2):365–367. doi: 10.1016/0014-5793(78)80788-1. [DOI] [PubMed] [Google Scholar]
- Schütte H., Hummel W., Kula M. R. Purification and characterization of a nicotinamide adenine dinucleotide-dependent secondary alcohol dehydrogenase from Candida boidinii. Biochim Biophys Acta. 1982 Jun 16;716(3):298–307. doi: 10.1016/0304-4165(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Sheehan M. C., Bailey C. J., Dowds B. C., McConnell D. J. A new alcohol dehydrogenase, reactive towards methanol, from Bacillus stearothermophilus. Biochem J. 1988 Jun 15;252(3):661–666. doi: 10.1042/bj2520661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Stone C. L., Li T. K., Bosron W. F. Stereospecific oxidation of secondary alcohols by human alcohol dehydrogenases. J Biol Chem. 1989 Jul 5;264(19):11112–11116. [PubMed] [Google Scholar]
- Walter K. A., Bennett G. N., Papoutsakis E. T. Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol. 1992 Nov;174(22):7149–7158. doi: 10.1128/jb.174.22.7149-7158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Welch R. W., Rudolph F. B., Papoutsakis E. T. Purification and characterization of the NADH-dependent butanol dehydrogenase from Clostridium acetobutylicum (ATCC 824). Arch Biochem Biophys. 1989 Sep;273(2):309–318. doi: 10.1016/0003-9861(89)90489-x. [DOI] [PubMed] [Google Scholar]
- Wills C., Kratofil P., Londo D., Martin T. Characterization of the two alcohol dehydrogenases of Zymomonas mobilis. Arch Biochem Biophys. 1981 Sep;210(2):775–785. doi: 10.1016/0003-9861(81)90245-9. [DOI] [PubMed] [Google Scholar]
- Yan R. T., Chen J. S. Coenzyme A-acylating aldehyde dehydrogenase from Clostridium beijerinckii NRRL B592. Appl Environ Microbiol. 1990 Sep;56(9):2591–2599. doi: 10.1128/aem.56.9.2591-2599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R. T., Zhu C. X., Chen J. S. Oxidation product(s) in acetaldehyde reacts with NAD(P)H and interferes with assay of alcohol dehydrogenase. Anal Biochem. 1987 Aug 1;164(2):362–366. doi: 10.1016/0003-2697(87)90505-7. [DOI] [PubMed] [Google Scholar]
- Yan Run-Tao, Zhu Chang-Xi, Golemboski Christine, Chen Jiann-Shin. Expression of Solvent-Forming Enzymes and Onset of Solvent Production in Batch Cultures of Clostridium beijerinckii ("Clostridium butylicum"). Appl Environ Microbiol. 1988 Mar;54(3):642–648. doi: 10.1128/aem.54.3.642-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngleson J. S., Jones W. A., Jones D. T., Woods D. R. Molecular analysis and nucleotide sequence of the adh1 gene encoding an NADPH-dependent butanol dehydrogenase in the Gram-positive anaerobe Clostridium acetobutylicum. Gene. 1989 May 30;78(2):355–364. doi: 10.1016/0378-1119(89)90238-2. [DOI] [PubMed] [Google Scholar]
- al-Kassim L. S., Tsai C. S. Studies of NADP(+)-preferred secondary alcohol dehydrogenase from Thermoanaerobium brockii. Biochem Cell Biol. 1990 Jun;68(6):907–913. doi: 10.1139/o90-135. [DOI] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Hög J. O., Hedén L. O., Kaiser R., Fleetwood L., Larsson K., Lake M., Holmquist B., Holmgren A., Hempel J. cDNA and protein structure for the alpha subunit of human liver alcohol dehydrogenase. Biochemistry. 1986 May 6;25(9):2465–2470. doi: 10.1021/bi00357a026. [DOI] [PubMed] [Google Scholar]