Abstract
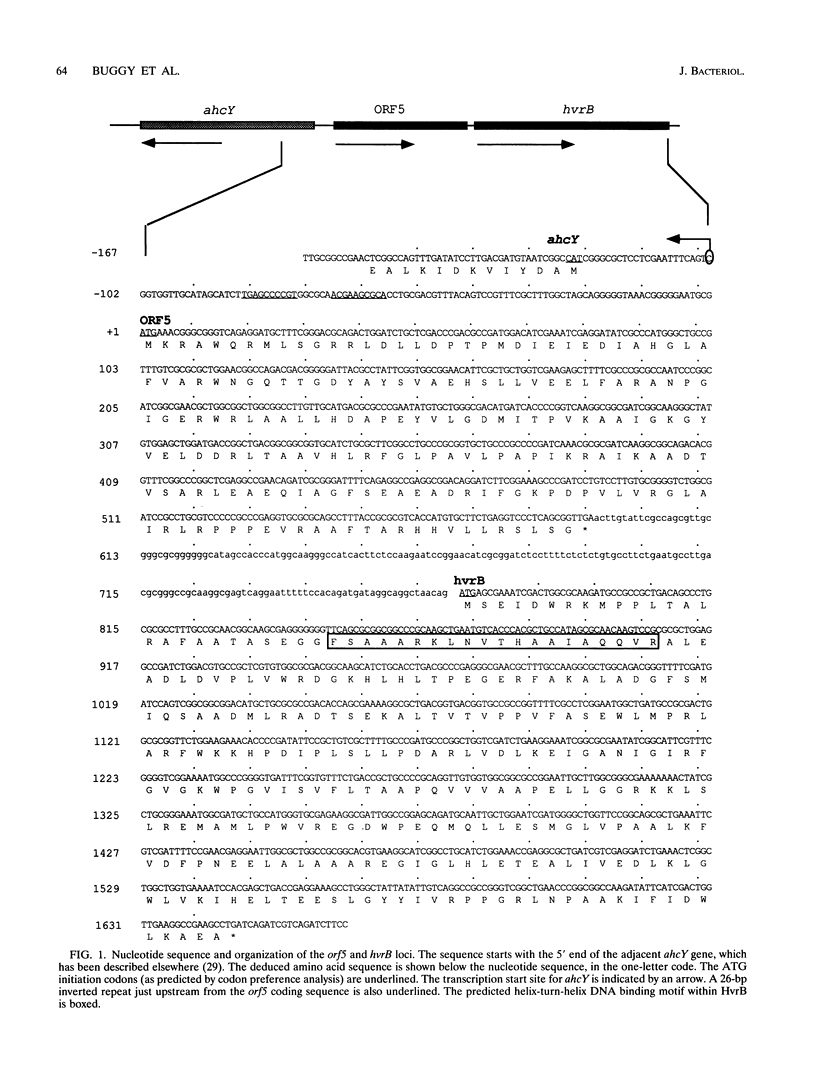
Here we present the nucleotide sequence and characterization of two genes, hvrB and orf5, that are located in the regulatory gene cluster from Rhodobacter capsulatus. The hvrB gene, which encodes a protein with a predicted molecular mass of 32 kDa, is shown to be highly homologous to genes encoding members of the LysR family of bacterial transcriptional regulators. A chromosomal disruption of hvrB is shown to result in the failure to regulate expression from the nearby ahcY and orf5 genes in response to alterations in light intensity. We show by primer extension mapping that the 5' end of ahcY-specific mRNA defines a promoter region exhibiting sequence similarity to known R. capsulatus promoter elements. Our mutational analysis further demonstrates that hvrB autoregulates its own expression in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37(1-3):111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- Bauer C. E., Marrs B. L. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7074–7078. doi: 10.1073/pnas.85.19.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C., Buggy J., Mosley C. Control of photosystem genes in Rhodobacter capsulatus. Trends Genet. 1993 Feb;9(2):56–60. doi: 10.1016/0168-9525(93)90188-N. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Chang M., Hadero A., Crawford I. P. Sequence of the Pseudomonas aeruginosa trpI activator gene and relatedness of trpI to other procaryotic regulatory genes. J Bacteriol. 1989 Jan;171(1):172–183. doi: 10.1128/jb.171.1.172-183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coulter-Karis D. E., Hershfield M. S. Sequence of full length cDNA for human S-adenosylhomocysteine hydrolase. Ann Hum Genet. 1989 May;53(Pt 2):169–175. doi: 10.1111/j.1469-1809.1989.tb01781.x. [DOI] [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Johnston A. W. Nodulation of legumes by Rhizobium: the recognized root? Cell. 1986 Oct 24;47(2):153–154. doi: 10.1016/0092-8674(86)90436-8. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 2. The effects of ethionine and threonine. Biochem J. 1962 Jun;83:550–559. doi: 10.1042/bj0830550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
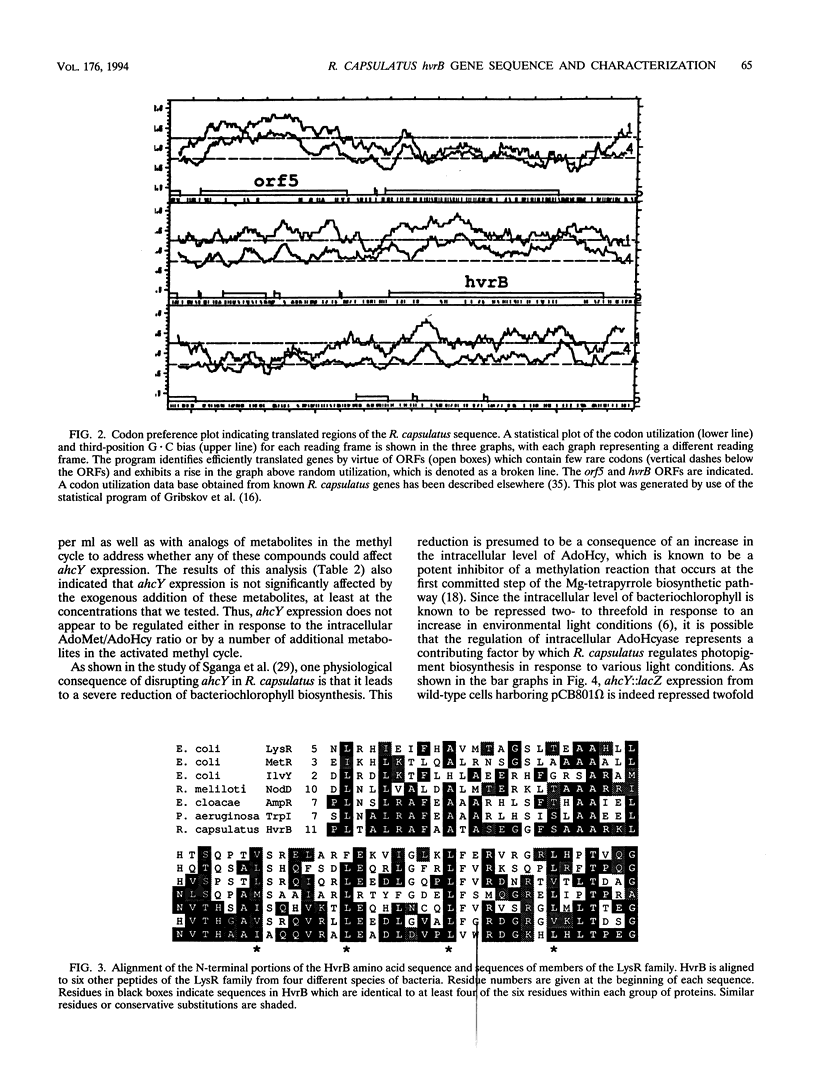
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
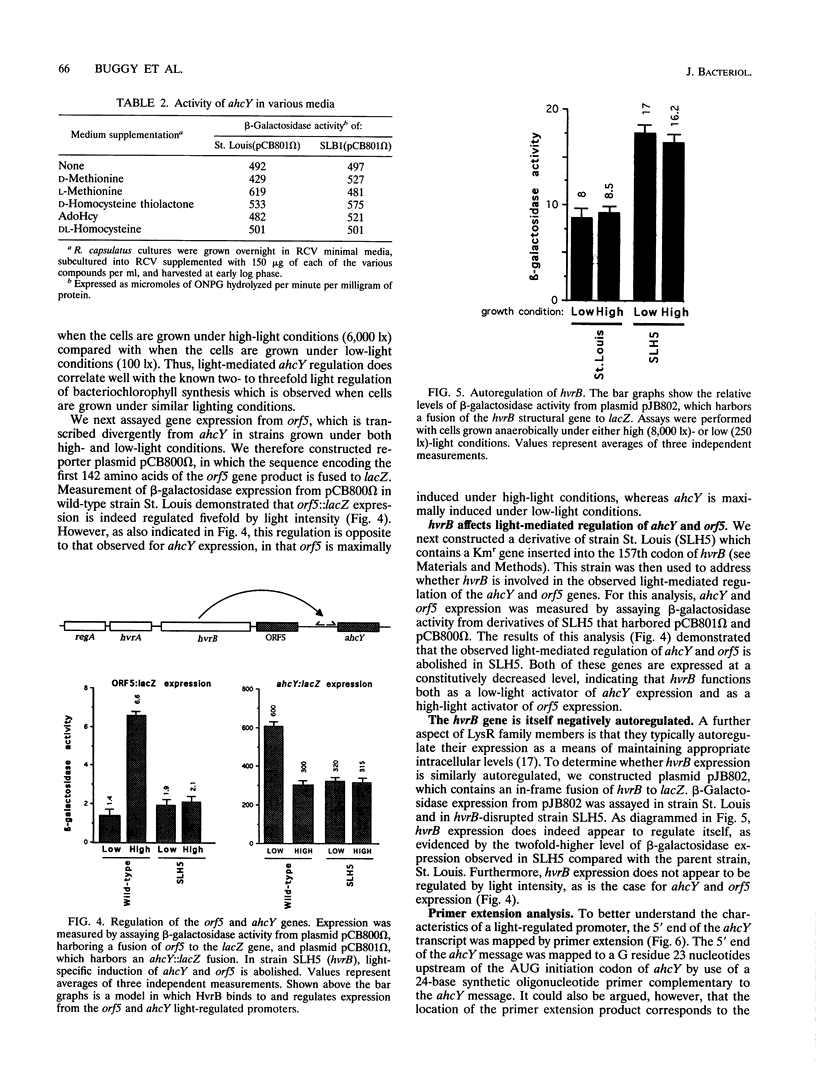
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasir J., Aksamit R. R., Backlund P. S., Jr, Cantoni G. L. Amino acid sequence of S-adenosyl-L-homocysteine hydrolase from Dictyostelium discoideum as deduced from the cDNA sequence. Biochem Biophys Res Commun. 1988 May 31;153(1):359–364. doi: 10.1016/s0006-291x(88)81231-2. [DOI] [PubMed] [Google Scholar]
- Lascelles J. The regulation of synthesis of iron and magnesium tetrapyrroles. Observations with mutant strains of Rhodopseudomonas spheroides. Biochem J. 1966 Jul;100(1):184–189. doi: 10.1042/bj1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Cook D. N., O'Brien D. A., Hearst J. E. Analysis of the promoter and regulatory sequences of an oxygen-regulated bch operon in Rhodobacter capsulatus by site-directed mutagenesis. J Bacteriol. 1993 Apr;175(7):2037–2045. doi: 10.1128/jb.175.7.2037-2045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Gomi T., Mueckler M. M., Fujioka M., Backlund P. S., Jr, Aksamit R. R., Unson C. G., Cantoni G. L. Amino acid sequence of S-adenosyl-L-homocysteine hydrolase from rat liver as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1987 Feb;84(3):719–723. doi: 10.1073/pnas.84.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. L., Abeles R. H. The mechanism of action of S-adenosylhomocysteinase. J Biol Chem. 1979 Feb 25;254(4):1217–1226. [PubMed] [Google Scholar]
- Plamann L. S., Stauffer G. V. Nucleotide sequence of the Salmonella typhimurium metR gene and the metR-metE control region. J Bacteriol. 1987 Sep;169(9):3932–3937. doi: 10.1128/jb.169.9.3932-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
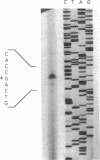
- Sganga M. W., Aksamit R. R., Cantoni G. L., Bauer C. E. Mutational and nucleotide sequence analysis of S-adenosyl-L-homocysteine hydrolase from Rhodobacter capsulatus. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6328–6332. doi: 10.1073/pnas.89.14.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sganga M. W., Bauer C. E. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell. 1992 Mar 6;68(5):945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer L. T., Plamann L. S., Stauffer G. V. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987 Apr;169(4):1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Young D. A., Bauer C. E., Williams J. C., Marrs B. L. Genetic evidence for superoperonal organization of genes for photosynthetic pigments and pigment-binding proteins in Rhodobacter capsulatus. Mol Gen Genet. 1989 Jul;218(1):1–12. doi: 10.1007/BF00330558. [DOI] [PubMed] [Google Scholar]