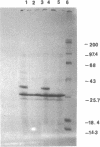
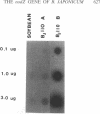
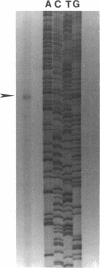
Abstract
The nodulation genes of rhizobia are regulated by the nodD gene product in response to host-produced flavonoids and appear to encode enzymes involved in the production of a lipo-chitose signal molecule required for infection and nodule formation. We have identified the nodZ gene of Bradyrhizobium japonicum, whose product is required for the addition of a 2-O-methylfucose residue to the terminal reducing N-acetylglucosamine of the nodulation signal. This substitution is essential for the biological activity of this molecule. Mutations in nodZ result in defective nodulation of siratro. Surprisingly, although nodZ clearly codes for nodulation function, it is not regulated by NodD and, indeed, shows elevated expression in planta. Therefore, nodZ represents a unique nodulation gene that is not under the control of NodD and yet is essential for the synthesis of an active nodulation signal.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Banfalvi Z., Kondorosi A. Production of root hair deformation factors by Rhizobium meliloti nodulation genes in Escherichia coli: HsnD (NodH) is involved in the plant host-specific modification of the NodABC factor. Plant Mol Biol. 1989 Jul;13(1):1–12. doi: 10.1007/BF00027330. [DOI] [PubMed] [Google Scholar]
- Banfalvi Z., Nieuwkoop A., Schell M., Besl L., Stacey G. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol Gen Genet. 1988 Nov;214(3):420–424. doi: 10.1007/BF00330475. [DOI] [PubMed] [Google Scholar]
- Bassam B. J., Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Identification of a nodD-dependent locus in the Rhizobium strain NGR234 activated by phenolic factors secreted by soybeans and other legumes. Mol Plant Microbe Interact. 1988 Apr;1(4):161–168. doi: 10.1094/mpmi-1-161. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanjuan J., Bhat U. R., Glushka J., Spaink H. P., Wijfjes A. H., van Brussel A. A., Stokkermans T. J., Peters N. K., Stacey G. The structures and biological activities of the lipo-oligosaccharide nodulation signals produced by type I and II strains of Bradyrhizobium japonicum. J Biol Chem. 1993 Aug 25;268(24):18372–18381. [PubMed] [Google Scholar]
- Demont N., Debellé F., Aurelle H., Dénarié J., Promé J. C. Role of the Rhizobium meliloti nodF and nodE genes in the biosynthesis of lipo-oligosaccharidic nodulation factors. J Biol Chem. 1993 Sep 25;268(27):20134–20142. [PubMed] [Google Scholar]
- Deshmane N., Stacey G. Identification of Bradyrhizobium nod genes involved in host-specific nodulation. J Bacteriol. 1989 Jun;171(6):3324–3330. doi: 10.1128/jb.171.6.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénarié J., Cullimore J. Lipo-oligosaccharide nodulation factors: a minireview new class of signaling molecules mediating recognition and morphogenesis. Cell. 1993 Sep 24;74(6):951–954. doi: 10.1016/0092-8674(93)90717-5. [DOI] [PubMed] [Google Scholar]
- Dénarié J., Debellé F., Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- Economou A., Hamilton W. D., Johnston A. W., Downie J. A. The Rhizobium nodulation gene nodO encodes a Ca2(+)-binding protein that is exported without N-terminal cleavage and is homologous to haemolysin and related proteins. EMBO J. 1990 Feb;9(2):349–354. doi: 10.1002/j.1460-2075.1990.tb08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Festl H., Ludwig W., Schleifer K. H. DNA hybridization probe for the Pseudomonas fluorescens group. Appl Environ Microbiol. 1986 Nov;52(5):1190–1194. doi: 10.1128/aem.52.5.1190-1194.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. M., Alvarez-Morales A., Hennecke H. The pleiotropic nature of symbiotic regulatory mutants: Bradyrhizobium japonicum nifA gene is involved in control of nif gene expression and formation of determinate symbiosis. EMBO J. 1986 Jun;5(6):1165–1173. doi: 10.1002/j.1460-2075.1986.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. Rhizobium--plant signal exchange. Nature. 1992 Jun 25;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Geiger O., Spaink H. P., Kennedy E. P. Isolation of the Rhizobium leguminosarum NodF nodulation protein: NodF carries a 4'-phosphopantetheine prosthetic group. J Bacteriol. 1991 May;173(9):2872–2878. doi: 10.1128/jb.173.9.2872-2878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis: detection of a protein factor in soybean root exudate which is involved in the nodulation process. Plant Physiol. 1984 Jan;74(1):84–89. doi: 10.1104/pp.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. Bacteriophage T4 late promoters: mapping 5' ends of T4 gene 23 mRNAs. EMBO J. 1982;1(1):107–114. doi: 10.1002/j.1460-2075.1982.tb01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop A. J., Banfalvi Z., Deshmane N., Gerhold D., Schell M. G., Sirotkin K. M., Stacey G. A locus encoding host range is linked to the common nodulation genes of Bradyrhizobium japonicum. J Bacteriol. 1987 Jun;169(6):2631–2638. doi: 10.1128/jb.169.6.2631-2638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J., Brierley H. L., Long S. R. Analysis of Rhizobium meliloti nodulation mutant WL131: novel insertion sequence ISRm3 in nodG and altered nodH protein product. J Bacteriol. 1991 May;173(10):3060–3065. doi: 10.1128/jb.173.10.3060-3065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. P., Relić B., Talmont F., Lewin A., Promé D., Pueppke S. G., Maillet F., Dénarié J., Promé J. C., Broughton W. J. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol Microbiol. 1992 Dec;6(23):3575–3584. doi: 10.1111/j.1365-2958.1992.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Quinto M., Bender R. A. Use of bacteriophage P1 as a vector for Tn5 insertion mutagenesis. Appl Environ Microbiol. 1984 Feb;47(2):436–438. doi: 10.1128/aem.47.2.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P., Debellé F., Maillet F., Lerouge P., Faucher C., Truchet G., Dénarié J., Promé J. C. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell. 1991 Dec 20;67(6):1131–1143. doi: 10.1016/0092-8674(91)90290-f. [DOI] [PubMed] [Google Scholar]
- Russell P., Schell M. G., Nelson K. K., Halverson L. J., Sirotkin K. M., Stacey G. Isolation and characterization of the DNA region encoding nodulation functions in Bradyrhizobium japonicum. J Bacteriol. 1985 Dec;164(3):1301–1308. doi: 10.1128/jb.164.3.1301-1308.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan J., Carlson R. W., Spaink H. P., Bhat U. R., Barbour W. M., Glushka J., Stacey G. A 2-O-methylfucose moiety is present in the lipo-oligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8789–8793. doi: 10.1073/pnas.89.18.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu A. K., Economou A., Hong G. F., Ghelani S., Johnston A. W., Downie J. A. Secretion of the Rhizobium leguminosarum nodulation protein NodO by haemolysin-type systems. Mol Microbiol. 1992 Jan;6(2):231–238. doi: 10.1111/j.1365-2958.1992.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Schwedock J., Long S. R. ATP sulphurylase activity of the nodP and nodQ gene products of Rhizobium meliloti. Nature. 1990 Dec 13;348(6302):644–647. doi: 10.1038/348644a0. [DOI] [PubMed] [Google Scholar]
- Smit G., Puvanesarajah V., Carlson R. W., Barbour W. M., Stacey G. Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABCSUIJ operon. J Biol Chem. 1992 Jan 5;267(1):310–318. [PubMed] [Google Scholar]
- So J. S., Hodgson A. L., Haugland R., Leavitt M., Banfalvi Z., Nieuwkoop A. J., Stacey G. Transposon-induced symbiotic mutants of Bradyrhizobium japonicum: isolation of two gene regions essential for nodulation. Mol Gen Genet. 1987 Apr;207(1):15–23. doi: 10.1007/BF00331485. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Aarts A., Stacey G., Bloemberg G. V., Lugtenberg B. J., Kennedy E. P. Detection and separation of Rhizobium and Bradyrhizobium Nod metabolites using thin-layer chromatography. Mol Plant Microbe Interact. 1992 Jan-Feb;5(1):72–80. doi: 10.1094/mpmi-5-072. [DOI] [PubMed] [Google Scholar]
- Spaink H. P. Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol Biol. 1992 Dec;20(5):977–986. doi: 10.1007/BF00027167. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Wijfjes A. H., Spaink H. P., Ruiz-Sainz J. E., Wijffelman C. A., Okker R. J., Lugtenberg B. J. nodO, a new nod gene of the Rhizobium leguminosarum biovar viciae sym plasmid pRL1JI, encodes a secreted protein. J Bacteriol. 1989 Dec;171(12):6764–6770. doi: 10.1128/jb.171.12.6764-6770.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]