Abstract
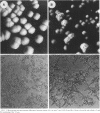
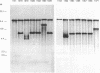
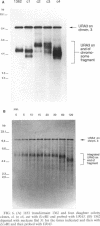
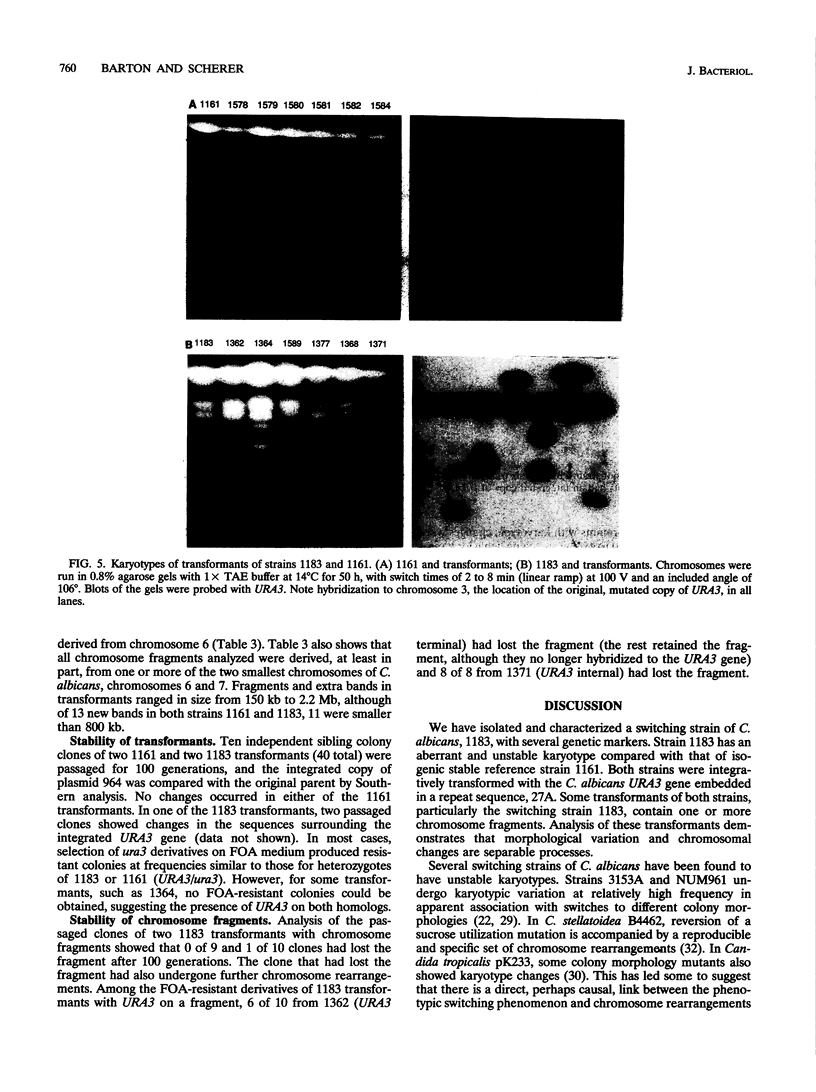
We have isolated a mutant of Candida albicans that switches between colony morphologies at high frequencies in a strain with several genetic markers. This strain, 1183, has an altered karyotype with two extra chromosomes. The 1183 karyotype is unstable upon passage. Using DNA transformation with the URA3 gene flanked by sequences from the C. albicans repeat sequence 27A, we have marked individual chromosomes of 1183 and 1161, a related smooth, stable strain. Many transformants contained one or more extra chromosomes, ranging in size from 150 kb to 2.1 Mb. Most were less than 800 kb and appeared to be fragments of a single chromosome. All fragments tested derive from one of the two smallest chromosomes. Six of 13 fragments contained the URA3 gene. In some cases, URA3 was located at the end of a fragment with adjacent telomere repeats. The integrated copy of URA3 was unstable in some 1183 transformants. Our results suggest that 1183 has a mutation affecting genomic stability. A connection between karyotypic changes and morphologic variation has been suggested from studies of several C. albicans strains; however, we find that gross karyotypic and morphological changes are separable processes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura K., Iwaguchi S., Homma M., Sukai T., Higashide K., Tanaka K. Electrophoretic karyotypes of clinically isolated yeasts of Candida albicans and C. glabrata. J Gen Microbiol. 1991 Nov;137(11):2531–2538. doi: 10.1099/00221287-137-11-2531. [DOI] [PubMed] [Google Scholar]
- Barton R. C., Gull K. Isolation, characterization, and genetic analysis of monosomic, aneuploid mutants of Candida albicans. Mol Microbiol. 1992 Jan;6(2):171–177. doi: 10.1111/j.1365-2958.1992.tb01998.x. [DOI] [PubMed] [Google Scholar]
- Chu W. S., Magee B. B., Magee P. T. Construction of an SfiI macrorestriction map of the Candida albicans genome. J Bacteriol. 1993 Oct;175(20):6637–6651. doi: 10.1128/jb.175.20.6637-6651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W. S., Rikkerink E. H., Magee P. T. Genetics of the white-opaque transition in Candida albicans: demonstration of switching recessivity and mapping of switching genes. J Bacteriol. 1992 May;174(9):2951–2957. doi: 10.1128/jb.174.9.2951-2957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton S., Penn C. W. Biological attributes of colony-type variants of Candida albicans. J Gen Microbiol. 1989 Dec;135(12):3363–3372. doi: 10.1099/00221287-135-12-3363. [DOI] [PubMed] [Google Scholar]
- Gerring S. L., Connelly C., Hieter P. Positional mapping of genes by chromosome blotting and chromosome fragmentation. Methods Enzymol. 1991;194:57–77. doi: 10.1016/0076-6879(91)94007-y. [DOI] [PubMed] [Google Scholar]
- Gil C., Pomés R., Nombela C. A complementation analysis by parasexual recombination of Candida albicans morphological mutants. J Gen Microbiol. 1988 Jun;134(6):1587–1595. doi: 10.1099/00221287-134-6-1587. [DOI] [PubMed] [Google Scholar]
- Gorman J. A., Chan W., Gorman J. W. Repeated use of GAL1 for gene disruption in Candida albicans. Genetics. 1991 Sep;129(1):19–24. doi: 10.1093/genetics/129.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshorn A. K., Grindle S. M., Scherer S. Gene isolation by complementation in Candida albicans and applications to physical and genetic mapping. Infect Immun. 1992 Mar;60(3):876–884. doi: 10.1128/iai.60.3.876-884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshorn A. K., Scherer S. Genetic analysis of prototrophic natural variants of Candida albicans. Genetics. 1989 Dec;123(4):667–673. doi: 10.1093/genetics/123.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Thorburn P. C. Healing of broken linear dicentric chromosomes in yeast. Genetics. 1984 Feb;106(2):207–226. doi: 10.1093/genetics/106.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B., Kirsch D. R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987 Jan;7(1):199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Magee P. T. Electrophoretic karyotypes and chromosome numbers in Candida species. J Gen Microbiol. 1987 Feb;133(2):425–430. doi: 10.1099/00221287-133-2-425. [DOI] [PubMed] [Google Scholar]
- McEachern M. J., Hicks J. B. Dosage of the smallest chromosome affects both the yeast-hyphal transition and the white-opaque transition of Candida albicans WO-1. J Bacteriol. 1991 Dec;173(23):7436–7442. doi: 10.1128/jb.173.23.7436-7442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern M. J., Hicks J. B. Unusually large telomeric repeats in the yeast Candida albicans. Mol Cell Biol. 1993 Jan;13(1):551–560. doi: 10.1128/mcb.13.1.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow B., Anderson J., Wilson J., Soll D. R. Bidirectional stimulation of the white-opaque transition of Candida albicans by ultraviolet irradiation. J Gen Microbiol. 1989 May;135(5):1201–1208. doi: 10.1099/00221287-135-5-1201. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Stotz A., Scherf C. DNA of Saccharomyces cerevisiae. Methods Enzymol. 1991;194:169–182. doi: 10.1016/0076-6879(91)94014-4. [DOI] [PubMed] [Google Scholar]
- Rikkerink E. H., Magee B. B., Magee P. T. Genomic structure of Candida stellatoidea: extra chromosomes and gene duplication. Infect Immun. 1990 Apr;58(4):949–954. doi: 10.1128/iai.58.4.949-954.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P., Sherman F., Hicks J. B. Chromosomal rearrangements associated with morphological mutants provide a means for genetic variation of Candida albicans. J Bacteriol. 1990 Mar;172(3):1276–1283. doi: 10.1128/jb.172.3.1276-1283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P. Variations of Candida albicans electrophoretic karyotypes. J Bacteriol. 1991 Oct;173(20):6586–6596. doi: 10.1128/jb.173.20.6586-6596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Magee P. T. Genetics of Candida albicans. Microbiol Rev. 1990 Sep;54(3):226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kobayashi I., Kanbe T., Tanaka K. High frequency variation of colony morphology and chromosome reorganization in the pathogenic yeast Candida albicans. J Gen Microbiol. 1989 Feb;135(Pt 2):425–434. doi: 10.1099/00221287-135-2-425. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Miyamae Y., Ishida I. Variation of colony morphology and chromosomal rearrangement in Candida tropicalis pK233. J Gen Microbiol. 1991 Jan;137(1):161–167. doi: 10.1099/00221287-137-1-161. [DOI] [PubMed] [Google Scholar]
- Traver C. N., Klapholz S., Hyman R. W., Davis R. W. Rapid screening of a human genomic library in yeast artificial chromosomes for single-copy sequences. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5898–5902. doi: 10.1073/pnas.86.15.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickes B. L., Golin J. E., Kwon-Chung K. J. Chromosomal rearrangement in Candida stellatoidea results in a positive effect on phenotype. Infect Immun. 1991 May;59(5):1762–1771. doi: 10.1128/iai.59.5.1762-1771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickes B., Staudinger J., Magee B. B., Kwon-Chung K. J., Magee P. T., Scherer S. Physical and genetic mapping of Candida albicans: several genes previously assigned to chromosome 1 map to chromosome R, the rDNA-containing linkage group. Infect Immun. 1991 Jul;59(7):2480–2484. doi: 10.1128/iai.59.7.2480-2484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]